UPPSALA, Sweden, and WILMINGTON, Mass., March 28, 2008 /PRNewswire via COMTEX/ -- Use of a low profile PressureWire yields a more accurate assessment of translesion pressure gradients when compared to catheter-derived pressure gradient (CPG) measurements, according to a paper published in the Journal of Interventional Cardiology (2008; Vol. 20, Issue 1: 63-65).
The authors report that although CPG measurements derived from both a catheter and PressureWire correlated with anatomic stenosis, PressureWire gradient was more accurate in estimating the clinical significance of peripheral arterial lesions, thus reducing the risk of inappropriate intervention. The paper was based on a study of 20 lesions in 16 patients undergoing angiography for peripheral vascular disease.
Entitled "Physiologic Evaluation of Translesion Pressure Gradients in Peripheral Arteries: Comparison of PressureWire and Catheter-Derived Measurements," this study, conducted at the Beth Israel Deaconess Medical Center in Boston, is the first to assess this hypothesis in patients with peripheral arterial occlusive disease.
full article >> http://www.foxbusiness.com/article/use-pressurewirer-yields-accurate-assessment-translesion-pressure-gradients_537830_1.html
Saturday, March 29, 2008
Use of PressureWire(R) Yields a More Accurate Assessment of Translesion Pressure Gradients in Peripheral Vasculature, Two New Studies Report
Posted by
www.med-centric.com
at
2:20 PM
0
comments
![]()
![]()
Tuesday, March 25, 2008
Cambridge Consultants: Groundbreaking Platform Allows Medical Devices to Communicate Wirelessly
US$10 Platform Lets Consumers Effortlessly Monitor Health at Home
CAMBRIDGE, England & BOSTON--(BUSINESS WIRE)--Cambridge Consultants today announced the first demonstration of the emerging industry standards for medical device interoperability. The 'Vena' platform is a breakthrough software solution on a single chip that allows medical devices such as blood pressure monitors to transmit data wirelessly. The development gives consumers, especially those with chronic conditions, the ability to monitor their own health accurately, systematically and independently. This platform uses low-cost wireless technology and will allow devices to deliver medical readings to a central monitor located in the home, or even to an online health record such as Google Health or Microsoft Health Vault. The Vena software solution can be added to a medical device using hardware with a potential cost of less than US $10 at the appropriate volumes and could even be available in medical devices by the end of 2008.
full article >> http://www.devicespace.com/news_story.aspx?NewsEntityId=90420
Posted by
www.med-centric.com
at
7:29 PM
0
comments
![]()
![]()
Monday, March 24, 2008
Hospitals Reuse Medical Devices To Lower Costs
In a bid to save costs and stem a rising tide of medical waste, hospitals are recycling a growing number of medical devices labeled as single-use, from scissors and scrubs to the sharp blades surgeons use to saw through bones.
Recycling medical devices labeled for single use is legal as long as certain Food and Drug Administration guidelines are followed. But the practice, which involves shipping devices to reprocessing facilities to be cleaned, sterilized and tested for reuse, has raised concerns about safety. Medical device makers say their single-use products are just that, and pose a higher risk of failure and harm when recycled. Reprocessing companies, hospital associations and environmental groups counter that the devices they reprocess are as safe as new thanks to modern sterilization methods, cost 40% to 60% less, and can eliminate thousands of tons of waste from landfills.
In January, after reviewing eight years of FDA data, the Government Accountability Office weighed in with a report concluding there is no evidence that reprocessed single-use devices create an elevated health risk for patients. About 100 devices -- just 2% of all devices labeled for single use -- are now reprocessed.
full article >> http://online.wsj.com/article/SB120588469924246975.html?mod=googlenews_wsj
Posted by
www.med-centric.com
at
7:07 AM
0
comments
![]()
![]()
FDA calls Medtronic drug pump warning Class I
LOS ANGELES (Reuters) - Medtronic Inc said on Wednesday that U.S. regulators classified its move to inform physicians about an increase in the rate of inflammatory mass cases in patients receiving drugs through the company's implantable infusion pumps as a Class I recall.
The medical device maker said no deaths have been associated with the problem and the recall classification does not change recommendations made to physicians in January.
The Food and Drug Administration defines a Class I recall as a situation in which there is a reasonable probability that use of the product will cause injury or death.
According to the FDA Web site, a medical device recall does not always mean that patients or doctors must stop using the product or return it to the company. A recall sometimes means the medical device needs to be checked, adjusted or fixed.
Medtronic said in a statement that it sent an update on January 16 to inform clinicians of an increase in the rate of inflammatory mass cases reported in patients using its SynchroMed and IsoMed infusion systems. The masses developed near the tip of the catheter attached to the pumps, which are typically used to dispense opioids for pain.
full article >> http://www.reuters.com/article/healthNews/idUSN1938924220080320
Posted by
www.med-centric.com
at
7:04 AM
0
comments
![]()
![]()
E-Z-EM, Inc. Stockholders Approve Merger Agreement
LAKE SUCCESS, N.Y.--(HSMN NewsFeed)--E-Z-EM, Inc. (NASDAQ: EZEM ) announced today that the stockholders of the Company voted to adopt the merger agreement providing for the acquisition of the Company by Bracco Diagnostics Inc. (Bracco), the US-based subsidiary of Bracco Imaging S.p.A and part of the Bracco Group, at a special meeting of the stockholders held yesterday, Thursday March 20, 2008, in Garden City, NY. The number of shares voting to adopt the merger agreement represents approximately 69.1% of the total number of shares outstanding and entitled to vote.
The proposed merger was announced on October 30, 2007 and is expected to close on or about April 1, 2008, pending the satisfaction or waiver of all the closing conditions set forth in the merger agreement. Under the terms of the merger agreement, Company stockholders will receive $21.00 per share in cash, without interest.
Posted by
www.med-centric.com
at
7:01 AM
0
comments
![]()
![]()
Synthetic heparin could be next step
The pursuit of a synthetic version of heparin, free of animal materials and made with stricter quality controls, is gaining more attention as awareness grows that the blood thinner can be sourced from an unregulated supply chain that starts with hog lots in rural China. "The reason we are pushing for the synthetic is that you can completely control the production process," said Jian Liu, associate professor of medicinal chemistry and natural products at the University of North Carolina School of Pharmacy, who is developing a synthetic heparin that is years from the U.S. market. "For the time being, we are stuck with the pig stuff. It has served us well for 50 years, but it was only a matter of time until something like this happened. It is too easy for the heparin extraction process to be contaminated if strict controls are not maintained."
The synthetic process purifies the drug and its ingredients every step of the way in laboratories, in contrast to the need for scrutiny of village workshops and farms in China that are now under investigation by U.S. and Chinese health officials.
full article >> http://www.chicagotribune.com/business/chi-sat-baxter-heparin-bax-mar22,0,3420880.story
Posted by
www.med-centric.com
at
6:58 AM
0
comments
![]()
![]()
PLC Systems Receives FDA Approval To Commence Pivotal Study Of RenalGuard(TM) In The U.S.
PLC Systems Inc. (Amex: PLC) announced that it has received conditional approval from the U.S. Food and Drug Administration (FDA) to begin enrollment in a U.S. pivotal trial to study the effectiveness of the Company's RenalGuard Therapy(TM) and RenalGuard System(TM) in the prevention of Contrast-Induced Nephropathy (CIN).Mark R. Tauscher, President and Chief Executive Officer of PLC, said, "We are very pleased with the FDA's conditional approval to the Investigational Device Exemption (IDE) supplement we filed last month. This will enable us to commence our pivotal study on schedule this spring. We are focused now on incorporating FDA's input into our final study protocol, and rapidly moving through the next phase of our clinical trial plan, which includes securing approvals to begin our study from hospital Institutional Review Boards at the sites that have elected to participate. FDA approval of the IDE is another major milestone on our path forward to commercializing the RenalGuard therapy and system, and we are very excited about beginning the pivotal trial."
full article >> http://www.medicalnewstoday.com/articles/101309.php
Posted by
www.med-centric.com
at
6:54 AM
0
comments
![]()
![]()
Thursday, March 20, 2008
Cook Medical Receives FDA 510(k) Clearance for Celect(TM) Vena Cava Filter and Gunther Tulip(TM) Vena Cava Filter Retrieval Set
Clearance Provides More Options for Prevention of Recurrent Pulmonary Embolism
BLOOMINGTON, Ind.--(HSMN NewsFeed)--Cook Medical, a global leader in the implantable vena cava (IVC) filter market, was granted 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Celect™ Vena Cava Filter. Cook’s Celect Filter is intended for the prevention of recurrent pulmonary embolism (PE) via placement in the vena cava and now can be optionally retrieved when clinically indicated.
Full text >>
Posted by
www.med-centric.com
at
8:12 PM
0
comments
![]()
![]()
Teleflex Medical Introduces the Next Step in Chronic Hemodialysis Catheter Technology -- the Arrow(R) NextStep(TM)
RESEARCH TRIANGLE PARK, N.C.--(HSMN NewsFeed)--Teleflex Medical, a leading global supplier of disposable products for critical care and surgical applications, has announced the NextStep Chronic Hemodialysis Catheter is available for release in the United States. The Arrow NextStep will debut at the Society of Interventional Radiology (SIR) Conference in Washington DC.
The Arrow NextStep is the first of its kind, a retrograde tunneled chronic hemodialysis catheter designed to combine a step-tip catheter’s ease of insertion and a split-tip’s sustained high flow. The Arrow NextStep’s unique tip is designed for a smooth transition through a sheath and for over-the-wire insertion.To reduce recirculation and deliver high flow, the Arrow NextStep’s tip has two unique complementary features: First, the ports on the catheter are reversed to take better advantage of blood flow dynamics. The venous port releases blood in the SVC and the arterial port draws blood from the right atrium. Second, the ports are significantly separated to enhance flow and minimize recirculation.
Full text >>
Posted by
www.med-centric.com
at
8:10 PM
0
comments
![]()
![]()
960-Patient Study Demonstrates Zero Blood Stream Infection In Patients Treated With Angiotech's Novel 5-FU Central Venous Catheter
Study Successfully Hit Primary End Point of Preventing Bacterial Colonization with a Trend Toward Superiority over the Market Leader
VANCOUVER, March 18 (HSMN NewsFeed) - Angiotech Pharmaceuticals, Inc. (NASDAQ: ANPI, TSX: ANP), a global specialty pharmaceutical and medical device company, announced today that the clinical data from its 960 patient clinical trial comparing its 5-Fluorouracil-coated (5-FU) Central Venous Catheter (CVC) with a chlorhexidine/silver sulfadiazine (CH-SS) coated CVC was presented by the clinical investigators at the 28th International Symposium on Intensive Care and Emergency Medicine (ISICEM) in Brussels, Belgium. Angiotech believes this study is the largest head-to-head coated CVC clinical trial ever completed. Based on the clinical trial data, the investigators concluded that Angiotech's 5-FU CVC met the primary endpoint of the study: non-inferior in its ability to prevent bacterial colonization of the catheter tip when compared to catheters coated with CH-SS. The rate of colonization of the 5-FU CVC was 2.9% (n=12), compared to 5.3% (n=21) in the CH-SS coated catheters (relative reduction in colonization with 5-FU coating of 46%, p=0.055).
Full text >>
Posted by
www.med-centric.com
at
8:08 PM
0
comments
![]()
![]()
Nuvelo Announces Phase 2 SONOMA-3 Trial Did Not Meet Target Product Profile and Discontinues Alfimeprase Development
SAN CARLOS, Calif., March 17 (HSMN NewsFeed) -- Nuvelo, Inc. (Nasdaq: NUVO ) today announced that data from the Phase 2 program in catheter occlusion (CO), known as SONOMA-3 (Speedy Opening of Non-functional and Occluded catheters with Mini-dose Alfimeprase), did not show sufficient improvement in catheter opening at the higher dose and concentration evaluated in the study to meet the desired target product profile. As a result, Nuvelo has ended further clinical development of alfimeprase including its programs in CO and acute ischemic stroke.
Full text >>
Posted by
www.med-centric.com
at
8:05 PM
0
comments
![]()
![]()
Judge Rules in Favor of Cook in Patent Infringement Suit
BLOOMINGTON, Ind.--The United States District Court for the Northern District of California granted summary judgment in favor of Cook Incorporated rejecting claims filed by Edwards Lifesciences LLC alleging Cook had infringed on Edwards’ patents for endovascular devices to treat aneurysms, Cook officials announced today.
press release >> http://www.insideindianabusiness.com/newsitem.asp?ID=28457
Posted by
www.med-centric.com
at
7:59 PM
0
comments
![]()
![]()
Treatment Gives Lung Cancer Patients With Inoperable Tumors Two Years Or More, Study Shows
Posted by
www.med-centric.com
at
7:55 PM
0
comments
![]()
![]()
Wednesday, March 19, 2008
Angiotech Showcases Its Hemostream(TM) Dialysis Catheter At The Society Of Interventional Radiology (SIR) Annual Meeting
Angiotech Pharmaceuticals, Inc. (NASDAQ: ANPI, TSX: ANP) announced that it will introduce and exhibit its innovative HemoStream(TM) chronic dialysis catheter at the 2008 Society of Interventional Radiology (SIR) Annual Scientific Meeting to be held in Washington, DC from March 15-18, 2008."It's exciting to participate at SIR and showcase the addition of another 'best-in-class' device offered by Angiotech. We also anticipate that the HemoStream(TM) could be a great candidate for our combination drug-device technologies, such as Angiotech's innovative 5-FU anti-infective platform," said George Leondis, General Manager, Angiotech Interventional.
Some of the potential benefits of the HemoStream(TM) catheter include:- Patented Triple Arterial Lumen Design: Ensures functional flow rates in the event that two lumens become completely occluded.- Transition: Provides atraumatic over-the-wire insertion without the need for a peel-away sheath.- 360 degree Arterial Tip Configuration: Eliminates catheter "side- walling" against vessel.
In April 2007, Angiotech entered into an agreement with Rex Medical, LP, which granted Angiotech an exclusive license to market and distribute the HemoStream(TM) catheter worldwide. The U.S. Food and Drug Administration (FDA) has given clearance to begin marketing the HemoStream(TM) chronic dialysis catheter in the United States.
full article >> http://www.medicalnewstoday.com/articles/100588.php
Posted by
www.med-centric.com
at
11:07 AM
0
comments
![]()
![]()
Angiotech and Rex Medical announce exclusive licensing and distribution agreement for the "Option(TM)" inferior vena cava filter
VANCOUVER, March 13 /PRNewswire-FirstCall/ - Angiotech Pharmaceuticals, Inc. announced today that it has entered into a definitive licensing agreement with privately held Rex Medical, LP ("Rex Medical") for exclusive worldwide rights to market and distribute the Option(TM) inferior vena cava (IVC) filter, a medical device that is implanted into the body's inferior vena cava to prevent pulmonary embolism (PE).
According to industry research, the U.S. market for IVC filters in 2007 was approximately $200 million with retrievable filters accounting for approximately two-thirds of this market. The Option(TM) IVC filter, developed by Rex Medical, is an IVC filter specifically designed for long-term retrieval post device implantation and is expected to be approved for both permanent and retrievable indications.
full article >> http://money.cnn.com/news/newsfeeds/articles/prnewswire/TO21313032008-1.htm
Posted by
www.med-centric.com
at
11:05 AM
0
comments
![]()
![]()
Cordis Corporation Receives U.S. Food and Drug Administration Clearance for S.M.A.R.T. Nitinol Stent Transhepatic Biliary System for 120 mm and 150 mm
WASHINGTON - (Business Wire) Cordis Corporation announced today at the 33rd Annual Scientific Meeting of the Society of Interventional Radiology meeting it has received 510(k) marketing clearance from the U.S. Food and Drug Administration for the S.M.A.R.T.® Nitinol Stent Transhepatic Biliary System for lengths of 120 mm and 150 mm. These stents are indicated for use in the palliative treatment of malignant strictures in the biliary tree that can restrict the flow of digestive fluids and compromise digestion.
Available for the first time in the US in the new lengths, the stents have demonstrated accurate stent placement, which may decrease the need for additional stents to cover the full narrowing of the bile duct. The safety and effectiveness of this device for use in the vascular system have not been established.
Posted by
www.med-centric.com
at
11:00 AM
0
comments
![]()
![]()
SIR: RF Ablation Extends Survival in Lung Cancer
WASHINGTON, March 17 -- Radiofrequency ablation of small malignant lung lesions appears to offer survival of at least two years for nearly three-quarters of patients not suitable for surgery, a French researcher said here. In 244 patients, 70% were still alive two years after the procedure and 38.8% had no viable malignant lung tissue, according to Thierry de Baere, M.D., of Institut Gustave-Roussy, near Paris.
full article >> http://www.medpagetoday.com/MeetingCoverage/SIRMeeting/tb/8774
Posted by
www.med-centric.com
at
10:58 AM
0
comments
![]()
![]()
Catheter-Directed Thrombolysis Proves Safe in Cancer Patients With Deep Vein Thrombosis: Presented at SIR
WASHINGTON, DC -- March 17, 2008 -- Percutaneous catheter-directed thrombolysis (CDT) is safe for acute symptomatic deep venous thrombosis (DVT) both in cancer patients and in noncancer patients, researchers reported here at the Society of Interventional Radiology (SIR) 33rd Annual Scientific Meeting.Approximately 15% of cancer patients develop DVT, according to lead author Stephen R. Preece, BA, Interventional Radiology Applicant, Johns Hopkins School of Medicine, Baltimore, Maryland.Catheter-directed thrombolysis has been shown to result in rapid reduction of thrombus burden and symptoms, and to reduce the risk of recurrence. However, CDT is associated with greater risk of complications, such as major bleeding, than conventional anticoagulation approaches. Therefore, patients with malignancies are not considered to be good candidates for DVT trials, explained Dr. Preece.
full article >> http://www.docguide.com/news/content.nsf/news/852571020057CCF68525740F006272E7
Posted by
www.med-centric.com
at
9:03 AM
0
comments
![]()
![]()
First Patient Enrolled In SONIC I Registry Of OmniSonics Medical Technologies' OmniWave(TM) Endovascular System
OmniSonics Medical Technologies, Inc., a developer of advanced medical devices for use in the treatment of vascular disease, announced that it has enrolled its first patient in the SONIC I Clinical Registry. SONIC I is a prospective, multi-centered U.S. registry study of the company's OmniWave(TM) Endovascular System (OES) in patients undergoing percutaneous mechanical thrombectomy of acute deep vein thrombosis (DVT). All patients enrolled in the SONIC I are scheduled for follow-up at 30 days and 6 months following the OmniWave procedure. The first patient was enrolled by Daniel Clair, M.D., Chairman of Vascular Surgery at The Cleveland Clinic.
full article >> http://www.medicalnewstoday.com/articles/100869.php
Posted by
www.med-centric.com
at
8:59 AM
0
comments
![]()
![]()
Long-Term Data for 884 Patients Show Vertebroplasty for Osteoporotic Spinal Fractures Provides Dramatic Pain Relief, Greatly Decreases Disability
One’s ability to manage everyday life—such as washing, dressing or standing—was measured by the commonly used Oswestry Disability Questionnaire (ODQ), which was completed by patients before—and again one month after—vertebroplasty. The ODQ scores changed from an average of 69.3 percent +/- 13.5 to 18.8 percent +/- 6.9, showing a highly significant improvement in mobility.
Posted by
www.med-centric.com
at
8:55 AM
0
comments
![]()
![]()
UFE Highly Effective in Cases Where Focused Ultrasound to Treat Uterine Fibroids Failed
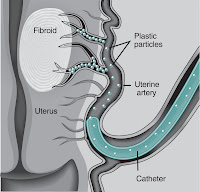 Newswise — Uterine fibroid embolization (UFE), a minimally invasive interventional radiology treatment for uterine fibroids, provides significant relief of symptoms for women whose focused ultrasound (FUS) treatment failed, according to a study released today during the Society of Interventional Radiology’s 33rd Annual Scientific Meeting in Washington, D.C. Primary care physicians and gynecologists can feel confident in informing patients that a failed FUS treatment does not require subsequent gynecological surgery. Those women can be successfully treated with UFE. Patients with a single large fibroid are candidates for FUS; patients with many fibroids, which is more often the case, would be better treated with UFE. There is limited long-term follow-up data for FUS and limited information on fibroid recurrence rates. In this retrospective study from Boston’s Brigham and Women’s Hospital, seven post-FUS patients who experienced therapeutic failure and were subsequently re-treated with UFE were reviewed. In all patients, their symptoms—such as heavy menstrual bleeding and the sensation of fullness or pressure in the lower abdomen—improved
Newswise — Uterine fibroid embolization (UFE), a minimally invasive interventional radiology treatment for uterine fibroids, provides significant relief of symptoms for women whose focused ultrasound (FUS) treatment failed, according to a study released today during the Society of Interventional Radiology’s 33rd Annual Scientific Meeting in Washington, D.C. Primary care physicians and gynecologists can feel confident in informing patients that a failed FUS treatment does not require subsequent gynecological surgery. Those women can be successfully treated with UFE. Patients with a single large fibroid are candidates for FUS; patients with many fibroids, which is more often the case, would be better treated with UFE. There is limited long-term follow-up data for FUS and limited information on fibroid recurrence rates. In this retrospective study from Boston’s Brigham and Women’s Hospital, seven post-FUS patients who experienced therapeutic failure and were subsequently re-treated with UFE were reviewed. In all patients, their symptoms—such as heavy menstrual bleeding and the sensation of fullness or pressure in the lower abdomen—improved
Posted by
www.med-centric.com
at
8:39 AM
0
comments
![]()
![]()
SIR: Percutaneous Cryoablation Effective Against Kidney Cancer
WASHINGTON, March 18 -- Percutaneous cryoablation stops kidney cancer cold, researchers said here. A year after treatment, between 94% and 97% of tumors treated by the minimally invasive technique had not recurred, according to two studies presented at the annual meeting of the Society of Interventional Radiology. The method, which involves freezing the heart of the tumor to about minus 150 degrees Celsius, should now be seen as a "curative minimally invasive treatment for kidney tumors," said Hussein Aoun, M.D., of Wayne State University in Detroit.
full article >> http://www.medpagetoday.com/MeetingCoverage/SIRMeeting/tb/8782
Posted by
www.med-centric.com
at
8:29 AM
0
comments
![]()
![]()
BTG's varicose vein treatment found safe in trial
LONDON, March 17 (Reuters) - British biotech firm BTG Plc's (BGC.L: Quote, Profile, Research) most important pipeline product, a varicose vein treatment called Varisolve, is safe for use in patients with a common heart defect, according to preliminary trial results.
In more than 90 percent of the 28 patients studied so far, tiny bubbles were detected in the blood during the treatment procedure but no neurological, visual or cardiac changes were observed, researchers said on Monday.
The U.S. Phase II safety study will continue until 50 patients have been treated and monitored.
The U.S. Food and Drug Administration requested the trial to see if there was any risk that microbubbles used in Varisolve -- an injectable foam made with carbon dioxide -- could pass into arteries and create problems in the brain.
full article >> http://www.reuters.com/article/rbssHealthcareNews/idUSL1489768220080317
Posted by
www.med-centric.com
at
8:24 AM
0
comments
![]()
![]()
Teleflex Medical Exhibits Arrow Pressure Injectable Acute Central Venous Catheters
RESEARCH TRIANGLE PARK, N.C. - (Business Wire) Teleflex Medical, a leading global supplier of disposable products for critical care and surgical applications, will exhibit its new pressure injectable central venous catheters at the Society of Interventional Radiology Meeting in Washington, D.C. from March 15 - 20. The FDA provided clearance to commercialize Arrow multi-lumen central venous catheters with the additional indication of pressure injection. Arrow is the only manufacturer of acute central venous catheters indicated for pressure injection up to 10ml/second.
Teleflex Medical estimates that nearly 20 percent of patients in acute care settings who are CVC recipients will need a CT scan. The additional indication gives clinicians who perform CT scans more options for scanning patients. CT technicians will now have the option of using an indwelling pressure injectable Arrow CVC without having to insert another catheter just for the scan. Arrow’s additional indication will reduce the stress on patients who up to now have had to endure another catheter insertion.
full article >> http://www.earthtimes.org/articles/show/teleflex-medical-exhibits-arrow-pressure-injectable-acute-central-venous-catheters,319800.shtml
Posted by
www.med-centric.com
at
8:17 AM
0
comments
![]()
![]()
Friday, March 14, 2008
Diomed files for Chapter 11, seeks sale to Biolitec
Diomed Holdings Inc. said Friday it, together with its wholly owned subsidiary, Diomed Inc., has filed a voluntary petition under Chapter 11 for bankruptcy protection.
The petition contemplates that Diomed will sell certain of its operating assets to Biolitec AG, a German-based manufacturer of medical lasers, optical fibers and other products. The move would enable Biolitec to continue to operate Diomed's business in the United States. Diomed develops and markets minimally invasive medical technologies, including a laser treatment for varicose veins.
Andover, Mass.-based Diomed (AMEX: DIO) said it has entered into a non-binding letter of intent with Biolitec for the sale of specified assets for a purchase price of between $6 and $7 million. Biolitec employs approximately 60 people its U.S. operations in East Longmeadow, Mass.
full article >> http://boston.bizjournals.com/boston/stories/2008/03/10/daily46.html?ana=yfcpc
Posted by
www.med-centric.com
at
5:19 PM
0
comments
![]()
![]()
Wednesday, March 12, 2008
Diomed Holdings Gets Delisting Determination Notice From AMEX
Diomed Holdings, Inc. (DIO) said Tuesday that it has received notice from the American Stock Exchange that the AMEX has determined to seek to delist the company's common stock on the basis that the company has not demonstrated a reasonable probability that it will regain compliance with the laid down standards for continued listing on the exchange. The standards require that a company maintain at least $4 million in stockholders' equity if the company has sustained losses from continuing operations in three of its four most recent fiscal years and at least $6 million in stockholders' equity if the company has sustained losses from continuing operations in its five most recent fiscal years.Diomed had previously submitted a compliance plan to the AMEX seeking to show its ability to regain compliance with these listing standards by the AMEX set February 3, 2009 deadline. In its notice, the AMEX also said it has determined that the low trading price of the company' common stock raises concern that the common stock may not be suitable for auction market trading, which would necessitate a reverse stock split within a reasonable period of time under certain section the AMEX Company Guide.
Posted by
www.med-centric.com
at
6:37 PM
0
comments
![]()
![]()
Medtronic Introduces Improvement to Minimally Invasive Treatment of Aortic Aneurysms in Europe
Hydrophilic Coating on Talent(R) Abdominal Stent Graft Aids Navigation of Medical Device through Tight and Tortuous Arteries
MINNEAPOLIS--(HSMN NewsFeed)--Continuing its record of innovation in endovascular therapies for aortic aneurysms, Medtronic, Inc. (NYSE: MDT ), today announced the European market launch of the Talent® Abdominal Stent Graft on the new Xcelerant® Hydro Delivery System, which features a hydrophilic coating designed to aid navigation of the device through tight and tortuous arteries by reducing friction with the artery wall.
full article >> http://salesandmarketingnetwork.com/news_release.php?ID=2023651
Posted by
www.med-centric.com
at
6:31 PM
0
comments
![]()
![]()
Bard Signs Agreement to Acquire Specialized Health Products International, Inc.
MURRAY HILL, N.J.--(HSMN NewsFeed)--C. R. Bard, Inc. (NYSE: BCR ) today announced that it has signed an agreement to acquire all the outstanding shares of Specialized Health Products International, Inc. (OTCBB: SHPI ) for a purchase price of $1.00 per share in cash, totaling approximately $68 million. Bard’s Access Systems subsidiary, located in Salt Lake City, Utah, will assume marketing responsibility for the related products. The company expects to complete the transaction following approval by the shareholders of Specialized Health Products and customary closing conditions, including Hart-Scott-Rodino clearance.
Specialized Health Products manufactures and markets vascular access products, including winged infusion sets, which are used to deliver therapeutic agents through vascular access ports. Many of its devices, including the SafeStep® Huber Needle Set, are designed to reduce the risk of accidental needlesticks for both patients and clinicians. Specialized Health Products is currently an original equipment supplier of winged infusion sets to Bard.
Full text >>
Posted by
www.med-centric.com
at
6:26 PM
0
comments
![]()
![]()
Medegen Introduces MaxPlus(R) Clear, First and Only Clear Positive Displacement Connector for Use in Infusion Therapy
Shown to Enhance Patient Care Leading to Lower Bloodstream Infection and Occlusion Rates
ONTARIO, Calif.--(HSMN NewsFeed)--Maximus, a business unit of leading infusion therapy firm Medegen Inc., today introduced its MaxPlus® Clear positive displacement connector for use in patient care. MaxPlus Clear provides complete visualization of the fluid path providing a visual reminder to completely perform clinical practices such as priming, disinfection, and flushing. This clarity enhances clinical practice which can ultimately reduce the occurrence of bloodstream infections and occlusions in patients receiving infusion therapy.
Maximus is the only medical device manufacturer to offer a clear positive displacement connector.
Full text >>
Posted by
www.med-centric.com
at
6:24 PM
0
comments
![]()
![]()
Genzyme Launches Renvela(R) in the U.S. for Dialysis Patients
Pursues Additional Approvals Globally for Dialysis and Earlier Stage Chronic Kidney Disease Patients
CAMBRIDGE, Mass., March 6 (HSMN NewsFeed) -- Genzyme Corp. (Nasdaq: GENZ ) today announced the U.S. launch of the phosphate binder Renvela® (sevelamer carbonate) for dialysis patients, as well as significant progress in its international efforts to secure additional approvals for the product.
Genzyme has submitted a marketing authorization application to the European Medicines Agency seeking approval of Renvela for the control of serum phosphorus in chronic kidney disease patients regardless of whether they are on dialysis. This application, which includes both tablet and powder formulations, must be validated before it will be accepted for review.
Full text >>
Posted by
www.med-centric.com
at
6:16 PM
0
comments
![]()
![]()
Thursday, March 6, 2008
Ovalum launches micro-catheter
Ovalum Ltd. has launched its TraVerse micro-catheter in the US. It is the firm's first product to be sold. TraVerse is part of the company's CiTOP CiTop Guidewire for peripheral vasculature applications, for which the US Food and Drug Administration (FDA) granted marketing approval in October 2007. CiTOP is used to open blocked arteries in the limbs.
full article >> http://www.globes.co.il/serveen/globes/docview.asp?did=1000317932&fid=942
Posted by
www.med-centric.com
at
10:49 PM
0
comments
![]()
![]()
Contaminant Found in Recalled Heparin
19 people are confirmed dead from allergic reactions associated to heparin use and 785 adverse reports have been linked to heparin since January of last year.
Baxter Healthcare identified the contaminant through sophisticated nuclear magnetic resonance spectroscopy and finds the fake heparin is similar to the blood thinner in its molecular makeup, but not identical.
The company doesn’t know if the contamination is accidental or intentional but it has been found in some five to 20 percent of the active pharmaceuticals in some heparin.
It’s also not known if the contaminant is what has led to so many injuries but the heparin lots associated with illnesses were all found to have the contaminant. What is also still undetermined is whether the Chinese plant is the source of the contamination.
full article >> http://www.injuryboard.com/national-news/contaminant-found-in-recalled-heparin.aspx?googleid=29934
Posted by
www.med-centric.com
at
10:41 PM
0
comments
![]()
![]()
Major Medical Journal Reports Higher Success, Fewer Complications and Lower Cost Treating DVT With Trellis(R) Isolated Thrombolysis
Majority of DVT Cases Treated in Single-Setting With Trellis-8 Catheter From Bacchus Vascular, Reducing Patient Lytic Exposure, Bleeding Complications and Treatment Cost
SANTA CLARA, Calif., March 6 (HSMN NewsFeed) -- Bacchus Vascular Inc., a leading provider of innovative medical devices used by interventional radiologists, vascular surgeons and interventional cardiologists for the minimally invasive treatment of deep vein thrombosis (DVT) and other peripheral vascular disease, announced today that the Journal of Vascular Interventional Radiology (JVIR) has published a study comparing the clinical use of the Trellis-8 infusion catheter from Bacchus Vascular with conventional catheter-directed thrombolysis (CDT) in the treatment of DVT in its March 2008 issue.
Results of the study showed that Grade II and III lysis was achieved in 93% of patients treated with the Trellis catheter and 79% of patients treated with CDT even though thrombolytic doses and infusion durations were less with the Trellis catheter than with conventional CDT. Major hemorrhage was reported in none of the Trellis catheter patients and in 8.5% of patients treated with CDT. The per-patient cost of therapy was $3,697 for the Trellis catheter and $5,473 for CDT. This cost reduction is due to approximately 80% of the Trellis catheter patients being treated in the single-setting of the interventional suite with less time required for follow-up monitoring in a costly critical care unit compared with CDT patients.
Full text >>
Posted by
www.med-centric.com
at
10:29 PM
0
comments
![]()
![]()
Cardinal Health Agrees to Acquire ChloraPrep(R) Manufacturer for $490 Million
Expands Offerings to Help Hospitals Prevent Infections
DUBLIN, Ohio, March 4 (HSMN NewsFeed) -- Cardinal Health, a global provider of products and services that improve the safety and productivity of health care, today announced a definitive agreement to acquire the assets of privately held Enturia Inc. for $490 million. The cash transaction includes Enturia's leading line of infection prevention products sold under the ChloraPrep® brand name and is expected to close within 60 days, subject to customary regulatory approvals and other conditions.
Full text >>
Posted by
www.med-centric.com
at
10:16 PM
0
comments
![]()
![]()
Sunday, March 2, 2008
Hatch Medical to Broker Chronic Total Occlusion Device
DULUTH, Ga.--(HSMN NewsFeed)--Hatch Medical, L.L.C., a medical device incubator and technology brokerage firm, announced today that it has recently entered into an agreement with Medical Miracles (Leeds, UK) to broker its patented and CE Marked chronic total occlusion (CTO) device, POLAR™.
POLAR™ (Path Of Least Arterial Resistance) is a mechanical device that generates reciprocal and lateral movements at the distal end of standard guidewires with frequencies of 16 to 100 Hz. when passed through an angioplasty balloon catheter, allowing clinicians to confidently cross occluded vessels.
Full text >>
Posted by
www.med-centric.com
at
11:13 PM
0
comments
![]()
![]()
First Patient Enrolled in Study Evaluating Performance of Spectranetics Laser Ablation Followed by GORE VIABAHN(R) Endoprosthesis...
Physician-sponsored study to examine combination therapy for lower limb in-stent restenosis in patients treated for Peripheral Vascular Disease
FLAGSTAFF, Ariz. & COLORADO SPRINGS, Colo.--(HSMN NewsFeed)--W. L. Gore & Associates, Inc. (Gore) and Spectranetics (NASDAQ: SPNC ) today announced the enrollment of the first two patients in the VIVA II: SALVAGE Trial. The patients were treated by Dr. Eric Dippel at Midwest Cardiovascular Research in Davenport, Iowa. The physician-sponsored SALVAGE Trial, is designed to evaluate the safety and performance of the GORE VIABAHN® Endoprosthesis with Heparin Bioactive Surface and the Spectranetics TURBO-Booster® and TURBO elite® laser catheter with the CVX-300® Excimer Laser System for the treatment of peripheral vascular disease (PVD) in the superficial femoral artery (SFA). Specifically, the study will evaluate the effectiveness of this combination therapy as a treatment for patients with chronic lower-limb ischemia associated with femoro-popliteal in-stent restenosis.
Full text >>
Posted by
www.med-centric.com
at
11:03 PM
0
comments
![]()
![]()


