AngioDynamics Inc. expects to close on its purchase of certain assets of Diomed Holdings, in the United State and the United Kingdom, on or about June 16.
The U.S. Bankruptcy Court in Boston gave its approval for AngioDynamics' deal for U. S. assets of Diomed and its wholly-owned subsidiary, Diomed Inc. on June 3. Diomed, which is based in Andover, Mass., filed for Chapter 11 bankruptcy protection in March.
AngioDynamics (Nasdaq: ANGO), a medical device maker based in Queensbury, had agreed in April to pay $8 million in cash for the U.S. assets, and $3 million in cash for certain assets of Diomed Ltd in the United Kingdom.
full article >> http://albany.bizjournals.com/albany/stories/2008/06/02/daily46.html?ana=yfcpc
Monday, June 9, 2008
AngioDynamics' purchase of certain Diomed Holdings set to close this month
Posted by
www.med-centric.com
at
9:51 AM
0
comments
![]()
![]()
Analysts mixed on patent victory's impact on Vnus
Analysts mixed on impact of Vnus patent victory; Piper Jaffray downgrades to "Neutral"
NEW YORK (AP) -- While medical device maker Vnus Medical Technologies Inc. claimed a major patent litigation victory Tuesday against two rivals, analysts are mixed over the victory's effect on the company's future performance.
Under a settlement announced Tuesday with AngioDynamics Inc. and Vascular Solutions Inc, the two companies will pay Vnus a total of $9.9 million in back royalties. The two companies were also granted a license to use Vnus products in the treatment of varicose veins.
While boosting his price target $1 to $21, Piper Jaffray analyst Thomas J. Gunderson Sr. cut his rating on Vnus shares to "Neutral" from "Buy," noting the stock has had a 46 percent run-up year-to date. He also expects revenue growth to slow.
Posted by
www.med-centric.com
at
9:48 AM
0
comments
![]()
![]()
MedCentric blog back up and running!
Posted by
www.med-centric.com
at
9:41 AM
0
comments
![]()
![]()
Tuesday, April 22, 2008
IDev Technologies, Inc. Closes $25 Million Financing
HOUSTON - (Business Wire) IDev Technologies, Inc., an emerging leader in the development and marketing of minimally invasive stent systems for the treatment of peripheral vascular and non-vascular diseases, today announced that it has closed a $25 million Series C round of financing. The financing was secured entirely by existing investors which include: PTV Lifesciences, Rivervest Venture Partners, Bay City Capital, Heron Capital, members of the senior management team, corporate advisors to the company and initial independent investors.
Thomas M. Tully, Chairman and CEO commented, “We are very pleased to have completed this round of financing as we enter into the full commercialization phase of the SUPERA® Transhepatic Biliary System. We also continue to be steadfast in our clinical trial and regulatory initiatives. The unanimous support from our existing investor base is just one indicator of the exceptional business opportunity that exists for this organization.”
full article >> http://www.earthtimes.org/articles/show/idev-technologies-inc-closes-25-million-financing,361087.shtml
Posted by
www.med-centric.com
at
10:37 PM
0
comments
![]()
![]()
Angiotech elects to suspend Vascular Wrap(TM) pivotal clinical trials
VANCOUVER, April 21 (HSMN NewsFeed) - Angiotech Pharmaceuticals, Inc. (NASDAQ: ANPI, TSX: ANP), a global specialty pharmaceutical and medical device company, today announced that it has elected to suspend enrolment in its U.S. and EU human clinical trials for its Vascular Wrap product candidate in patients undergoing surgery for hemodialysis access, pending a safety review to evaluate an imbalance of infections that have been observed between the two study groups. The U.S. and EU trials each consist of two study groups; (1) patients who received the graft/Vascular Wrap combination; and, (2) patients who received the graft alone.
Full text >>
Posted by
www.med-centric.com
at
10:30 PM
0
comments
![]()
![]()
Friday, April 18, 2008
CoolTouch Introduces CoolBlue Duet(TM) Laser Lipolysis Suction Handpiece
Reduces Lipolysis Procedure Time by Half
ROSEVILLE, Calif., April 18 /PRNewswire/ -- CoolTouch Inc., a
pioneering manufacturer of medical lasers, announced today the introduction
of their new CoolBlue Duet(TM) laser lipolysis suction handpiece set
designed specifically to be used with the CoolLipo(TM) 1320 laser system.
Using a standard liposuction cannula, ablation with aspiration and skin
tightening are accomplished all in one action, which dramatically reduces
the time of the laser lipolysis procedure.
"The CoolBlue Duet suction handpiece doubles the speed of laser
lipolysis and liposuction with better results and increased patient
satisfaction," states Robert Weiss, MD, FACPh, Associate Clinical Professor
of Dermatology at Johns Hopkins University School of Medicine.
FDA cleared for laser-assisted lipolysis and general dermatological
surgery, the CoolLipo 1320 laser procedure improves the outcome of
conventional liposuction in small areas such as the neck, chin and arms and
now is fast enough to be effectively used on larger areas such as the
abdomen and thighs. The excellent tissue absorption properties of the 1320
nm wavelength tighten the skin by directly targeting collagen and
connective tissue. The CoolLipo procedure is performed in a physician's
office under local anesthesia. Patient benefits include minimal, if any
bruising, and less recovery time and cost than a face or neck lift.
full article >> http://www.prnewswire.com/cgi-bin/stories.pl?ACCT=104&STORY=/www/story/04-18-2008/0004795480&EDATE=
Posted by
www.med-centric.com
at
6:50 AM
1 comments
![]()
![]()
FDA Approves Medtronic's Talent(TM) Abdominal Stent Graft for Repairing Aortic Aneurysms
Innovative Medical Device Enables Minimally-Invasive Treatment of Potentially Fatal Condition
MINNEAPOLIS--(HSMN NewsFeed)--Medtronic, Inc. (NYSE: MDT ), today announced approval from the U.S. Food and Drug Administration for use of the Talent™ Abdominal Stent Graft on the CoilTrac Delivery System, an innovative medical device that expands patient access to a minimally-invasive treatment option for abdominal aortic aneurysms.
Present in an estimated 1.2 million people and responsible for approximately 15,000 annual deaths in the United States, an abdominal aortic aneurysm (AAA) is a dangerous bulge or weakening of the body’s main artery that can rupture with fatal consequences if left untreated. Ruptured AAAs are currently the 10th leading cause of death among U.S. men over age 55, with fewer than 20 percent of people surviving a rupture. But early detection through painless ultrasound screening and minimally invasive treatment with a technique called endovascular repair (EVAR) have historically shown a significant improvement in the survival rate for patients of all ages.
Full text >>
Posted by
www.med-centric.com
at
6:25 AM
0
comments
![]()
![]()
Angiotech's novel 5-FU Central Venous Catheter receives FDA 510(k) clearance
Angiotech's novel 5-FU Central Venous Catheter receives FDA 510(k) clearance
VANCOUVER, April 17 (HSMN NewsFeed) - Angiotech Pharmaceuticals, Inc. (NASDAQ: ANPI, TSX: ANP), a global specialty pharmaceutical and medical device company, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its innovative 5-Fluorouracil-coated (5-FU) Central Venous Catheter (CVC) in the United States.
"The 5-FU CVC represents our first drug-eluting medical device product to be researched and developed completely in-house by Angiotech's R & D and clinical teams, without the aid of a corporate partner," said Dr. William Hunter, President and CEO of Angiotech. "This is an important milestone in our Company's history, and we look forward to moving into the commercial phase of our 5-FU CVC product, as well as developing other implantable devices that utilize this novel and proprietary anti-infective technology platform."
Full text >>
Posted by
www.med-centric.com
at
6:23 AM
0
comments
![]()
![]()
Tuesday, April 15, 2008
Pathway Medical Technologies, Inc. Secures $24.5 Million Financing
KIRKLAND, Wash., April 15 (HSMN NewsFeed) --Pathway Medical Technologies, Inc., an innovator of endovascular treatments for peripheral arterial disease (PAD), announced today that it has successfully secured a $24.5 million Series C-1 round of financing. The financing was obtained from existing investors HLM Venture Partners, Latterell Venture Partners, Oxford Bioscience Partners, Forbion Capital Partners, Giza Venture Capital and individual investors.
Pathway Medical Technologies, Inc. was founded to design, market and manufacture medical devices for the treatment of arterial disease. The company's initial focus is treating peripheral arterial disease (PAD) more quickly and effectively than existing technologies. An estimated 8-12 million people are afflicted by PAD in the U.S., and that number is projected to grow to over 20 million during the next 10 years. The company's Pathway PV(TM) Atherectomy System allows for a minimally invasive procedure designed to restore circulation in the peripheral arteries by removing both hard and soft diseased tissue. For further information, visit the company's Web site at www.pathwaymedical.com .
Full text >>
Posted by
www.med-centric.com
at
9:05 PM
0
comments
![]()
![]()
Medical Ventures Announces FDA Application Update for Metricath Gemini
RICHMOND, BRITISH COLUMBIA--(Marketwire - April 15, 2008) - MEDICAL VENTURES CORP. (TSX VENTURE:MEV), a medical devices company specializing in products for the vascular and surgical markets, provides the following progress update on its pre-market approval (PMA) application for the Metricath Gemini® dual-balloon angioplasty catheter:- The Company received an interim response to its PMA application from the U.S. Food & Drug Administration. The response requests additional information related to clinical and non-clinical aspects of the application that was submitted in Q4 2007. Medical Ventures is assembling the requested information with the assistance of the investigational sites that participated in the GAAME (Gemini Angioplasty and Arterial Measurement Evaluation) clinical trial and is targeting to submit this information to the FDA in June.
full article >> http://www.marketwire.com/mw/release.do?id=843829
Posted by
www.med-centric.com
at
9:00 PM
0
comments
![]()
![]()
Sirtex Receives FDA Approval for FAST Clinical Trial
WILMINGTON, Mass. - (Business Wire) Sirtex, a leading developer of targeted and innovative cancer therapies, has received FDA approval under Investigational Device Exemption to conduct the first clinical trial to evaluate the safety of concurrent administration of FOLFOX6 and bevacizumab, an anti-angiogenic, with Selective Internal Radiation Therapy (SIRT) using SIR-Spheres®1 microspheres (FAST) as first-line treatment of patients with colorectal cancer that has metastasized to the liver. Dennis L. Carter, M.D., radiation oncologist, and Charles Nutting, D.O., interventional radiologist, will serve as principal investigators for the FAST trial.
It is estimated that 70 percent of colorectal cancers will metastasize to the liver, and less than 10 percent of liver metastases can be surgically removed. Without any treatment, the median survival after detection of liver metastases is approximately nine months, depending on the extent of the disease.
“Although SIR-Spheres microspheres are FDA approved for colorectal liver metastases, SIRT is typically used in the United States much later in the treatment course when patients have exhausted other options,” says Dr. Samuel Putnam, medical director of U.S. operations for Sirtex. “However, there is a great deal of data suggesting that combining SIR-Spheres microspheres with newer chemotherapies and biologics earlier may improve survival rates and the patient’s quality of life. This study marks an important step in determining the best way to utilize these combination therapies for treating advanced liver tumors.”
full article >> http://www.earthtimes.org/articles/show/sirtex-receives-fda-approval-for-fast-clinical-trial,353238.shtml
Posted by
www.med-centric.com
at
8:58 PM
0
comments
![]()
![]()
BSD Medical Submits 510(k) Premarket Notification for MicroThermX®-100 Microwave Ablation System
A 510(k) Premarket Notification includes documentation regarding compliance to applicable standards (UL, IEC, EMC, EMI, etc.), demonstration of substantial equivalence to predicate devices, documentation of required testing—including biocompatibility, shelf life, sterilization, software, hardware, and other required performance testing and documentation. The company’s 510(k) submission involves 1,251 pages.
BSD Medical’s product line includes systems that have been designed strategically to offer a complete range of thermal treatment products using microwave and RF (radiofrequency) technology. BSD is the leading developer of hyperthermia systems. Hyperthermia is used to treat certain types of cancer using heat to increase the effectiveness of companion radiation therapy. The MicroThermX-100 Microwave Ablation System represents an expansion of BSD’s products into new applications, as the MicroThermX-100 is designed as a stand-alone therapy that is used to ablate diseased tissue using heat alone.
press release >> http://www.businesswire.com/portal/site/google/?ndmViewId=news_view&newsId=20080415005407&newsLang=en
Posted by
www.med-centric.com
at
8:51 PM
0
comments
![]()
![]()
Ultrasound-accelerated thrombolysis "safe and effective" in DVT
Ultrasound-accelerated thrombolysis is more effective for DVT lysis than thrombolysis alone, a small US study has suggested [1]. This relatively new technique involves the delivery of low-intensity ultrasound energy alongside thrombolytic agent delivery into the occluded vessel.The study authors evaluated the clinical outcomes of ultrasound-accelerated thrombolysis in 47 patients with 53 cases of DVT, including lower and upper extremity and hepatic locations.They reported partial lysis in 91 per cent, with complete lysis of the occlusion in seven out of ten patients. Major complications were seen in just two patients who developed a hematoma at the site of earlier surgery.The group concluded: "The present… study demonstrates a considerable improvement versus previous studies of catheter-directed thrombolysis alone for the treatment of DVT, with fewer complications, reduced drug doses, and shorter infusion times.
full article >> http://www.medicexchange.com/mall/departmentpage.cfm/MedicExchangeUSA/_81675/4297/departments-contentview
Posted by
www.med-centric.com
at
8:47 PM
0
comments
![]()
![]()
Friday, April 11, 2008
HealthTrust Purchasing Group Awards Contract to Arrow Central Venous Catheter Line
RESEARCH TRIANGLE PARK, N.C.--(HSMN NewsFeed)--HealthTrust Purchasing Group, LP has announced a new contract award to Teleflex Medical for Arrow® Central Venous Catheters. The new agreement began April 1, 2008 and extends through December 31, 2010.
The Arrow brand offers one of the industry’s most comprehensive ranges of central venous catheters and related products: from ARROWg+ard® impregnated catheters and uncoated catheters to scores of comprehensive kits that include an array of options, such as large barrier drape and safety needles. Arrow recently introduced the industry’s first pressure injectable central venous catheter rated to 10 mL/sec, which is included on the HealthTrust agreement.
Full text >>
Posted by
www.med-centric.com
at
11:03 PM
0
comments
![]()
![]()
Covidien Unveils the Next Generation in Advanced SurgeryLigaSure Advance(TM) Instrument for Laparoscopic Procedures
BOULDER, Colo.--(HSMN NewsFeed)--Covidien (NYSE: COV, BSX: COV), a leading global provider of healthcare products, today announced that its Energy-based Devices business unit has introduced LigaSure Advance™, the first multifunctional laparoscopic instrument that offers surgeons fingertip control of both tissue fusion and monopolar dissection.
LigaSure Advance™ was created in response to what surgeons said they need to optimize efficiency and safety in the operating room. This latest innovation in the LigaSure™ lineup is a 5 mm hand-activated instrument that provides surgeons with the ability to create fusion cycles in two to four seconds, and when used with Valleylab™ mode, the unique energy modality delivers dissection and hemostasis.With sterile-field control of mode and power settings, LigaSure Advance™ is designed to enable surgeons and perioperative staff to focus more effectively on the procedure and patient. Its versatility reduces the need for instrument exchanges, making procedures more efficient and potentially reducing overall operating time.
full article >> http://salesandmarketingnetwork.com/news_release.php?ID=2024124
Posted by
www.med-centric.com
at
11:01 PM
0
comments
![]()
![]()
Microwave Treatments For Enlarged Prostate Cause Blood Pressure Surges, Study Shows
ScienceDaily (Apr. 11, 2008) — Many men who receive microwave therapy for enlarged prostates experience significant surges in blood pressure that could raise their risk of a heart attack or stroke, according to new research findings published recently in Mayo Clinic Proceedings.
The Mayo Clinic-led study of 185 consecutive patients who received transurethral microwave therapy at four medical centers found that 42 percent experienced systolic blood pressure surges of more than 30 mm Hg, while 5 percent had surges of more than 70 mm Hg.
"Men who are candidates for this minimally invasive microwave therapy tend also to be at higher risk for cardiac events," says Lance Mynderse, M.D., the Mayo Clinic urologist who authored the study. "Blood pressure surges of the magnitude identified in this study are troubling side effects of treatment that need to be monitored and managed."
full article >> http://www.sciencedaily.com/releases/2008/04/080408105820.htm
Posted by
www.med-centric.com
at
10:58 PM
0
comments
![]()
![]()
AngioDynamics Pursues Asset Purchase Agreements With Diomed Holdings, Inc.
QUEENSBURY, N.Y.--(BUSINESS WIRE)--AngioDynamics (NASDAQ: ANGO - News), a leading provider of innovative medical devices used by interventional radiologists, nephrologists and surgeons for the minimally invasive treatment of cancer and peripheral vascular disease, today announced that it has entered into asset purchase agreements with Diomed Holdings, Inc., Diomed, Inc. and Diomed Limited for the acquisition of certain assets of Diomed’s business in the United States and United Kingdom. The agreement with Diomed Holdings, Inc. and Diomed, Inc. is subject to an auction process administered by the bankruptcy court, as a result of Diomed's Chapter 11 bankruptcy proceedings, which commenced on March 14, 2008, and other customary closing conditions.
AngioDynamics has agreed to pay $8 million in cash for the assets of Diomed’s U.S. business engaged in the sale of systems for the endovenous laser treatment of varicose veins. In addition, AngioDynamics has agreed to pay $3 million in cash for the assets of Diomed’s U.K. business based in Cambridge, England. Diomed’s U.K. operations manufacture and distribute systems used in the endovenous laser treatment of varicose veins and was placed under the control of a U.K. Insolvency Administrator on March 14, 2008. The offer to purchase Diomed’s U.S. assets is conditioned upon AngioDynamics’ purchase of Diomed’s U.K. assets. The offer to purchase Diomed’s U.K. assets is conditioned upon the entry of an order in the U.S. bankruptcy court authorizing the purchase of the U.S. assets.
press release >> http://biz.yahoo.com/bw/080410/20080410006210.html?.v=1
Posted by
www.med-centric.com
at
10:54 PM
0
comments
![]()
![]()
Vascular Solutions Settles Diomed Case
Vascular Solutions Settles Diomed Patent Case for $3.58 Million
NEW YORK (Associated Press) - Medical device manufacturer Vascular Solutions Inc. said Wednesday it will pay $3.58 million to settle Diomed Holdings Inc.'s patent lawsuit.
In March 2007 a federal jury in Massachusetts awarded Diomed $4.1 million. Vascular said it recorded a $5 million litigation provision in the 2007 first quarter, which was later raised to $5.2 million because of interest on the jury's award.
full article >> http://money.cnn.com/news/newsfeeds/articles/apwire/75721e0e41ee93df1e66c002b95d00d9.htm
Posted by
www.med-centric.com
at
10:52 PM
0
comments
![]()
![]()
Tuesday, April 8, 2008
Gore Revise Study Receives Approval from FDA
FLAGSTAFF, Ariz.--(BUSINESS WIRE)--FDA approval to proceed with the Gore REVISE (VasculaR AccEss ReVision with Viabahn® EndoproSthesis vs PercutanEous Transluminal Angioplasty) Study was announced today by W. L. Gore & Associates (Gore). The Gore REVISE Study is a randomized, multi-center clinical trial intended to establish efficacy and safety of the GORE VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface to revise arterio-venous grafts at the venous anastomosis in hemodialysis patients. The study will randomize patients to the GORE VIABAHN® Endoprosthesis and to percutaneous transluminal angioplasty (PTA).
Tom Vesely, MD, Medical Director on the Gore REVISE Study and an Interventional Radiologist at Vascular Access Center in Frontenac Grove, Mo., states, “The study is designed to demonstrate the clinical benefit of using a stent-graft for AV graft revisions, rather than PTA alone.” Dr. Vesely continues, “With a focus on target-lesion primary patency, the study is designed to show an increased amount of time between interventions in dialysis access grafts.”
press release >> http://www.businesswire.com/portal/site/google/?ndmViewId=news_view&newsId=20080407006421&newsLang=en
Posted by
www.med-centric.com
at
8:40 PM
0
comments
![]()
![]()
NKF: HCV Outbreaks at Dialysis Units Linked to Outmoded Infection Control
DALLAS, April 7 -- Four outbreaks of hepatitis C at dialysis centers were caused by infection control practices that disregarded CDC recommendations, according to an investigation by the agency. Each outbreak was associated with at least two and as many as six practices that did not conform to CDC recommendations for infection control at dialysis centers, the agency's Nicola Thompson, Ph.D., reported at the National Kidney Foundation's spring clinical meetings here. Practices commonly associated with HCV infection at dialysis centers include re-use of IV medication vials and contamination of equipment and the environment by use of mobile carts and inadequate cleaning, she noted.
full article >> http://www.medpagetoday.com/MeetingCoverage/NKF/tb/9027
Posted by
www.med-centric.com
at
8:36 PM
0
comments
![]()
![]()
Sixty-Two Died After Allergic Reactions to Heparin
April 8 (Bloomberg) -- Sixty-two people given the blood- thinner heparin died since January 2007 after suffering allergic reactions or low blood pressure, up from 19 previously reported, U.S. regulators said.
The symptoms were consistent with those that led to a recall of Baxter International Inc.'s version of heparin, Food and Drug Administration spokeswoman Karen Riley said today after the data were posted on the agency's Web site. Riley couldn't immediately say how many of the deaths occurred in patients who used Baxter's version of the drug.
The agency increased the toll from the 19 reported last month after reviewing additional information, Riley said. The FDA hasn't determined whether the reported deaths are the result of a contaminant that has been identified in Baxter's heparin. Regulators are investigating whether the cheaper ingredient was added to raw heparin from China accidentally or intentionally to boost profits.
full article >> http://www.bloomberg.com/apps/news?pid=20601087&sid=a2cDgR2LlfdA&refer=home
Posted by
www.med-centric.com
at
7:43 PM
0
comments
![]()
![]()
Vascular Solutions Wins $4.5 Million Jury Verdict in Litigation With Marine Polymer Technologies
MINNEAPOLIS, April 8, 2008 (HSMN NewsFeed) -- Vascular Solutions, Inc. (NasdaqGM:VASC ) today announced that the jury has returned a verdict in its favor in the litigation against Marine Polymer Technologies, Inc. for product disparagement concerning statements made regarding the safety of Vascular Solutions' D-Stat Dry hemostat product. In its verdict the jury found that Marine Polymer's statements were false and disparaged the D-Stat Dry product and awarded Vascular Solutions $4.5 million in monetary damages. The jury rejected Marine Polymer's counterclaims in their entirety. This verdict is subject to customary post-trial motions in the United States Federal District Court for the District of Massachusetts and appeal.
Full text >>
Posted by
www.med-centric.com
at
7:35 PM
0
comments
![]()
![]()
Sunday, April 6, 2008
RF Ablation Plus Chemoembolization Comparable to Surgery for Early Liver Cancer
NEW YORK APR 02, 2008 (Reuters Health) - Radiofrequency ablation combined with chemoembolization provides comparable overall and disease-free survival as hepatectomy for early-stage hepatocellular carcinoma, Japanese investigators report.
In their study, the overall survival rate at 5 years was 75% with the combined treatment and 81% with surgery. The corresponding recurrence-free survival rates were also similar, 27% and 26%.
The findings suggest that "radiofrequency ablation combined with chemoembolization provides patients with early-stage hepatocellular carcinoma similar results to surgical intervention," lead author Dr. Koichiro Yamakado told Reuters Health. He added that his team is now planning to conduct a randomized trial to confirm this.
Surgical resection is considered the gold standard for treating early-stage disease, but only 9% to 29% of patients are eligible for this procedure either because of underlying pathology that limits their hepatic reserve or because tumor nodules are spread throughout the liver, according to the report in the April issue of Radiology.
full article >> http://www.cancerpage.com/news/article.asp?id=12068
Posted by
www.med-centric.com
at
9:41 AM
0
comments
![]()
![]()
Abraxis BioScience Initiates Patient Enrollment in Phase II Study of Coroxane(TM) for Prevention of Restenosis in Peripheral Vascular Disease
LOS ANGELES, Apr 02, 2008 (BUSINESS WIRE) -- Abraxis BioScience, Inc. (NASDAQ:ABII), a fully integrated biotechnology company, today announced it has initiated enrollment in its Phase II clinical trial to evaluate the efficacy and safety of Coroxane(TM: 97.98, -2.06, -2.05%) for the prevention and reduction of restenosis following revascularization of the superficial femoral artery (SFA).
Coroxane(TM: 97.98, -2.06, -2.05%) (nanometer-sized paclitaxel, ABRAXANE(R: 62.96, -1.36, -2.11%), under the trade name Coroxane(TM: 97.98, -2.06, -2.05%)) is a novel, solvent free, albumin-bound form of paclitaxel used for cardiovascular applications. ABRAXANE(R: 62.96, -1.36, -2.11%) is the first and only approved protein-bound nanometer-sized solvent-free taxane and is the first commercial product to validate Abraxis' proprietary nab(TM: 97.98, -2.06, -2.05%) technology platform. ABRAXANE(R: 62.96, -1.36, -2.11%) (for Injectable Suspension (paclitaxel protein-bound particles for injectable suspension) (albumin-bound)) is marketed for the treatment of breast cancer.
full article >> http://www.foxbusiness.com/markets/industries/health-care/article/abraxis-bioscience-initiates-patient-enrollment-phase-ii-study-coroxanetm_544886_10.html
Posted by
www.med-centric.com
at
9:37 AM
0
comments
![]()
![]()
BioSphere Medical Highlights Patient and Economic Benefits When Ob/Gyns and Interventional Radiologists Collaborate
ROCKLAND, Mass., Apr 03, 2008 (BUSINESS WIRE) -- BioSphere Medical, Inc. (NASDAQ: BSMD: 4.36, +0.05, +1.16%) today announced that more than 41,000 ob/gyns around the country are expected to receive a supplement in the April 2008 Contemporary OB/GYN which highlights the clinical benefits that result when ob/gyns and interventional radiologists (IRs) work in collaboration to treat women suffering with symptomatic uterine fibroids. The supplement, entitled "Expert Exchange: How to Formulate the Relationship Between the Ob/Gyn and the Interventional Radiologist for the Treatment of Uterine Fibroids," was funded by BioSphere Medical.
full article >> http://www.foxbusiness.com/markets/industries/health-care/article/biosphere-medical-highlights-patient-economic-benefits-ob2fgyns-interventional_547184_10.html
Posted by
www.med-centric.com
at
9:32 AM
0
comments
![]()
![]()
Update: New Venous Journal Articles
Perspect Vasc Surg Endovasc Ther. 2008 Apr 2 [Epub ahead of print]
Laser and Radiofrequency Endovenous Ablation of Venous Reflux.
Golan JF, Glenn DM.
North Shore Vascular Associates.
Endovenous modalities to treat superficial venous reflux of the lower extremities have revolutionized management of patients with varicose veins. Laser and radiofrequency probes have both found their way into the arsenal of physicians treating venous reflux. Although both offer distinct advantages and minor drawbacks, they each offer the convenience of in-office treatment, faster recovery, and improved safety over traditional surgical procedures. This article will briefly discuss the technique, treatment results, and potential complications associated with each procedure.
N Engl J Med. 2008 Apr 3;358(14):1525-6.
Links
Microembolism during foam sclerotherapy of varicose veins.
Ceulen RP, Sommer A, Vernooy K.
J Cardiovasc Surg (Torino). 2008 Feb;49(1):19-26.
Links
Options in the management of varicose veins, 2008.
Hirsch SA, Dillavou E.
Department of Vascular Surgery, School of Medicine, University of Pittsburgh, Pittsburgh, PA 15232, USA. hirssa@upmc.edu
Modern management of varicose veins requires knowledge of the principal procedures currently utilized, which are compression sclerotherapy, foam sclerotherapy, endovenous laser therapy, radiofrequency closure, microincision phlebectomy, transilluminated powered phlebectomy, radiofrequency closure of perforating veins, perforation invagination stripping, subfascial endoscopic perforator surgery, saphenous valvuloplasty and external laser therapy. A review of the above procedures is presented with a brief description of their indications, performance and results.
J Vasc Surg. 2008 Apr;47(4):901-2.
Links
Regarding "Light assisted stab phlebectomy: Report of a technique for removal of lower extremity varicose veins".
Samson RH.
Florida State University Medical School, Mote Vascular Foundation, Inc, Sarasota, Fla.
Posted by
www.med-centric.com
at
9:11 AM
0
comments
![]()
![]()
Wednesday, April 2, 2008
Boston Scientific Announces Sale of TriVascular Endovascular Aortic Repair Program
NATICK, Mass.-- Boston Scientific Corporation (NYSE: BSX ) today announced the sale of Boston Scientific Santa Rosa Corp., formerly known as TriVascular, Inc., to TV2 Holding Company, a privately held company based in Santa Rosa, California. Boston Scientific Santa Rosa Corp. holds equipment and intellectual property related to the TriVascular endovascular aortic repair (EVAR) program. Terms of the sale include $30 million in cash paid at closing to Boston Scientific and a warrant allowing Boston Scientific to purchase a minority interest in TV2.
Full text >>
Posted by
www.med-centric.com
at
10:39 PM
0
comments
![]()
![]()
Hydromer, Inc. Enters Into a Royalty Generating Coatings Supply Agreement for Neurovascular and Cardiovascular Catheters
BRANCHBURG, NJ, Apr 02, 2008 (MARKET WIRE via COMTEX) -- Hydromer, Inc. (OTCBB: HYDI) announces that it has entered into a two-year, self-renewing Supply and Support Agreement with an undisclosed medical device company.
Under the terms of this non-exclusive agreement, Hydromer will supply its proprietary and patented Hydromer(R: 64.79, +0.56, +0.87%) hydrophilic coatings for use on various Pebax(R: 64.79, +0.56, +0.87%) plastic neurovascular and cardiovascular catheters. This Hydromer coating system becomes slippery when made wet, improving the ease of catheter placement and removal by the physician during complicated procedures within the patient's vascular system.
full article >> http://www.foxbusiness.com/markets/industries/industrials/article/hydromer-enters-royalty-generating-coatings-supply-agreement-neurovascular_545537_6.html
Posted by
www.med-centric.com
at
10:17 PM
0
comments
![]()
![]()
Luminetx Launches GS Model
MEMPHIS, Tenn.--(BUSINESS WIRE)--Luminetx Corp. today announced it has launched the next generation of the VeinViewer − the VeinViewerGS. This “Global Standard” model was specifically designed to meet the rigorous quality requirements for the international marketplace.
From today forward, the GS model will be offered to both international and domestic clients as Luminetx’s flagship product. According to the company, this model was born as a result of the Luminetx global expansion strategy allowing Luminetx to market and sell the VeinViewer outside of the United States.
“Clinically speaking, this is the same technological powerhouse that’s been on the market for the last year,” said Chris Schnee, vice president of global marketing for Luminetx. “So our domestic clients can rest assured that their current device is still as leading-edge and as relevant as the GS model is today.”
full article >> http://www.businesswire.com/portal/site/google/?ndmViewId=news_view&newsId=20080401005218&newsLang=en
Posted by
www.med-centric.com
at
10:07 PM
0
comments
![]()
![]()
Cook Wins Injunction Against Competitor and Former Sales Representatives Over Violations of Employee Non-Compete Agreements
Court ruling also places limitations on Endologix’s hiring of Cook sales reps
BLOOMINGTON, Ind.--(BUSINESS WIRE)--Cook Medical has won a preliminary injunction against Endologix, Inc. and four former sales reps barring them from violating Cook’s non-compete agreement. The ruling, handed down March 25 in the United States District Court Southern District of Indiana, also enjoins Endologix, which manufactures a device to treat abdominal aortic aneurysm (AAA) that is a direct competitor to Cook’s global sales leading Zenith line of AAA endografts, from hiring Cook sales reps and placing them in their former territories and accounts.
“This ruling further supports Cook’s position that it is improper for competitors to ignore non-compete agreements that are fair and reasonable,” said Pete Yonkman, executive vice president for sales and marketing of Cook Medical. “In addition, we believe the practice of hiring a direct competitor’s sales representatives contributes to unnecessary escalations in health care costs. We spend a great amount of time and resources training our sales representatives to be valued partners in the delivery of quality health care to patients.”
press release >> http://www.businesswire.com/portal/site/google/?ndmViewId=news_view&newsId=20080331005197&newsLang=en
Posted by
www.med-centric.com
at
10:00 PM
0
comments
![]()
![]()
Saturday, March 29, 2008
Use of PressureWire(R) Yields a More Accurate Assessment of Translesion Pressure Gradients in Peripheral Vasculature, Two New Studies Report
UPPSALA, Sweden, and WILMINGTON, Mass., March 28, 2008 /PRNewswire via COMTEX/ -- Use of a low profile PressureWire yields a more accurate assessment of translesion pressure gradients when compared to catheter-derived pressure gradient (CPG) measurements, according to a paper published in the Journal of Interventional Cardiology (2008; Vol. 20, Issue 1: 63-65).
The authors report that although CPG measurements derived from both a catheter and PressureWire correlated with anatomic stenosis, PressureWire gradient was more accurate in estimating the clinical significance of peripheral arterial lesions, thus reducing the risk of inappropriate intervention. The paper was based on a study of 20 lesions in 16 patients undergoing angiography for peripheral vascular disease.
Entitled "Physiologic Evaluation of Translesion Pressure Gradients in Peripheral Arteries: Comparison of PressureWire and Catheter-Derived Measurements," this study, conducted at the Beth Israel Deaconess Medical Center in Boston, is the first to assess this hypothesis in patients with peripheral arterial occlusive disease.
full article >> http://www.foxbusiness.com/article/use-pressurewirer-yields-accurate-assessment-translesion-pressure-gradients_537830_1.html
Posted by
www.med-centric.com
at
2:20 PM
0
comments
![]()
![]()
Tuesday, March 25, 2008
Cambridge Consultants: Groundbreaking Platform Allows Medical Devices to Communicate Wirelessly
US$10 Platform Lets Consumers Effortlessly Monitor Health at Home
CAMBRIDGE, England & BOSTON--(BUSINESS WIRE)--Cambridge Consultants today announced the first demonstration of the emerging industry standards for medical device interoperability. The 'Vena' platform is a breakthrough software solution on a single chip that allows medical devices such as blood pressure monitors to transmit data wirelessly. The development gives consumers, especially those with chronic conditions, the ability to monitor their own health accurately, systematically and independently. This platform uses low-cost wireless technology and will allow devices to deliver medical readings to a central monitor located in the home, or even to an online health record such as Google Health or Microsoft Health Vault. The Vena software solution can be added to a medical device using hardware with a potential cost of less than US $10 at the appropriate volumes and could even be available in medical devices by the end of 2008.
full article >> http://www.devicespace.com/news_story.aspx?NewsEntityId=90420
Posted by
www.med-centric.com
at
7:29 PM
0
comments
![]()
![]()
Monday, March 24, 2008
Hospitals Reuse Medical Devices To Lower Costs
In a bid to save costs and stem a rising tide of medical waste, hospitals are recycling a growing number of medical devices labeled as single-use, from scissors and scrubs to the sharp blades surgeons use to saw through bones.
Recycling medical devices labeled for single use is legal as long as certain Food and Drug Administration guidelines are followed. But the practice, which involves shipping devices to reprocessing facilities to be cleaned, sterilized and tested for reuse, has raised concerns about safety. Medical device makers say their single-use products are just that, and pose a higher risk of failure and harm when recycled. Reprocessing companies, hospital associations and environmental groups counter that the devices they reprocess are as safe as new thanks to modern sterilization methods, cost 40% to 60% less, and can eliminate thousands of tons of waste from landfills.
In January, after reviewing eight years of FDA data, the Government Accountability Office weighed in with a report concluding there is no evidence that reprocessed single-use devices create an elevated health risk for patients. About 100 devices -- just 2% of all devices labeled for single use -- are now reprocessed.
full article >> http://online.wsj.com/article/SB120588469924246975.html?mod=googlenews_wsj
Posted by
www.med-centric.com
at
7:07 AM
0
comments
![]()
![]()
FDA calls Medtronic drug pump warning Class I
LOS ANGELES (Reuters) - Medtronic Inc said on Wednesday that U.S. regulators classified its move to inform physicians about an increase in the rate of inflammatory mass cases in patients receiving drugs through the company's implantable infusion pumps as a Class I recall.
The medical device maker said no deaths have been associated with the problem and the recall classification does not change recommendations made to physicians in January.
The Food and Drug Administration defines a Class I recall as a situation in which there is a reasonable probability that use of the product will cause injury or death.
According to the FDA Web site, a medical device recall does not always mean that patients or doctors must stop using the product or return it to the company. A recall sometimes means the medical device needs to be checked, adjusted or fixed.
Medtronic said in a statement that it sent an update on January 16 to inform clinicians of an increase in the rate of inflammatory mass cases reported in patients using its SynchroMed and IsoMed infusion systems. The masses developed near the tip of the catheter attached to the pumps, which are typically used to dispense opioids for pain.
full article >> http://www.reuters.com/article/healthNews/idUSN1938924220080320
Posted by
www.med-centric.com
at
7:04 AM
0
comments
![]()
![]()
E-Z-EM, Inc. Stockholders Approve Merger Agreement
LAKE SUCCESS, N.Y.--(HSMN NewsFeed)--E-Z-EM, Inc. (NASDAQ: EZEM ) announced today that the stockholders of the Company voted to adopt the merger agreement providing for the acquisition of the Company by Bracco Diagnostics Inc. (Bracco), the US-based subsidiary of Bracco Imaging S.p.A and part of the Bracco Group, at a special meeting of the stockholders held yesterday, Thursday March 20, 2008, in Garden City, NY. The number of shares voting to adopt the merger agreement represents approximately 69.1% of the total number of shares outstanding and entitled to vote.
The proposed merger was announced on October 30, 2007 and is expected to close on or about April 1, 2008, pending the satisfaction or waiver of all the closing conditions set forth in the merger agreement. Under the terms of the merger agreement, Company stockholders will receive $21.00 per share in cash, without interest.
Posted by
www.med-centric.com
at
7:01 AM
0
comments
![]()
![]()
Synthetic heparin could be next step
The pursuit of a synthetic version of heparin, free of animal materials and made with stricter quality controls, is gaining more attention as awareness grows that the blood thinner can be sourced from an unregulated supply chain that starts with hog lots in rural China. "The reason we are pushing for the synthetic is that you can completely control the production process," said Jian Liu, associate professor of medicinal chemistry and natural products at the University of North Carolina School of Pharmacy, who is developing a synthetic heparin that is years from the U.S. market. "For the time being, we are stuck with the pig stuff. It has served us well for 50 years, but it was only a matter of time until something like this happened. It is too easy for the heparin extraction process to be contaminated if strict controls are not maintained."
The synthetic process purifies the drug and its ingredients every step of the way in laboratories, in contrast to the need for scrutiny of village workshops and farms in China that are now under investigation by U.S. and Chinese health officials.
full article >> http://www.chicagotribune.com/business/chi-sat-baxter-heparin-bax-mar22,0,3420880.story
Posted by
www.med-centric.com
at
6:58 AM
0
comments
![]()
![]()
PLC Systems Receives FDA Approval To Commence Pivotal Study Of RenalGuard(TM) In The U.S.
PLC Systems Inc. (Amex: PLC) announced that it has received conditional approval from the U.S. Food and Drug Administration (FDA) to begin enrollment in a U.S. pivotal trial to study the effectiveness of the Company's RenalGuard Therapy(TM) and RenalGuard System(TM) in the prevention of Contrast-Induced Nephropathy (CIN).Mark R. Tauscher, President and Chief Executive Officer of PLC, said, "We are very pleased with the FDA's conditional approval to the Investigational Device Exemption (IDE) supplement we filed last month. This will enable us to commence our pivotal study on schedule this spring. We are focused now on incorporating FDA's input into our final study protocol, and rapidly moving through the next phase of our clinical trial plan, which includes securing approvals to begin our study from hospital Institutional Review Boards at the sites that have elected to participate. FDA approval of the IDE is another major milestone on our path forward to commercializing the RenalGuard therapy and system, and we are very excited about beginning the pivotal trial."
full article >> http://www.medicalnewstoday.com/articles/101309.php
Posted by
www.med-centric.com
at
6:54 AM
0
comments
![]()
![]()
Thursday, March 20, 2008
Cook Medical Receives FDA 510(k) Clearance for Celect(TM) Vena Cava Filter and Gunther Tulip(TM) Vena Cava Filter Retrieval Set
Clearance Provides More Options for Prevention of Recurrent Pulmonary Embolism
BLOOMINGTON, Ind.--(HSMN NewsFeed)--Cook Medical, a global leader in the implantable vena cava (IVC) filter market, was granted 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Celect™ Vena Cava Filter. Cook’s Celect Filter is intended for the prevention of recurrent pulmonary embolism (PE) via placement in the vena cava and now can be optionally retrieved when clinically indicated.
Full text >>
Posted by
www.med-centric.com
at
8:12 PM
0
comments
![]()
![]()
Teleflex Medical Introduces the Next Step in Chronic Hemodialysis Catheter Technology -- the Arrow(R) NextStep(TM)
RESEARCH TRIANGLE PARK, N.C.--(HSMN NewsFeed)--Teleflex Medical, a leading global supplier of disposable products for critical care and surgical applications, has announced the NextStep Chronic Hemodialysis Catheter is available for release in the United States. The Arrow NextStep will debut at the Society of Interventional Radiology (SIR) Conference in Washington DC.
The Arrow NextStep is the first of its kind, a retrograde tunneled chronic hemodialysis catheter designed to combine a step-tip catheter’s ease of insertion and a split-tip’s sustained high flow. The Arrow NextStep’s unique tip is designed for a smooth transition through a sheath and for over-the-wire insertion.To reduce recirculation and deliver high flow, the Arrow NextStep’s tip has two unique complementary features: First, the ports on the catheter are reversed to take better advantage of blood flow dynamics. The venous port releases blood in the SVC and the arterial port draws blood from the right atrium. Second, the ports are significantly separated to enhance flow and minimize recirculation.
Full text >>
Posted by
www.med-centric.com
at
8:10 PM
0
comments
![]()
![]()
960-Patient Study Demonstrates Zero Blood Stream Infection In Patients Treated With Angiotech's Novel 5-FU Central Venous Catheter
Study Successfully Hit Primary End Point of Preventing Bacterial Colonization with a Trend Toward Superiority over the Market Leader
VANCOUVER, March 18 (HSMN NewsFeed) - Angiotech Pharmaceuticals, Inc. (NASDAQ: ANPI, TSX: ANP), a global specialty pharmaceutical and medical device company, announced today that the clinical data from its 960 patient clinical trial comparing its 5-Fluorouracil-coated (5-FU) Central Venous Catheter (CVC) with a chlorhexidine/silver sulfadiazine (CH-SS) coated CVC was presented by the clinical investigators at the 28th International Symposium on Intensive Care and Emergency Medicine (ISICEM) in Brussels, Belgium. Angiotech believes this study is the largest head-to-head coated CVC clinical trial ever completed. Based on the clinical trial data, the investigators concluded that Angiotech's 5-FU CVC met the primary endpoint of the study: non-inferior in its ability to prevent bacterial colonization of the catheter tip when compared to catheters coated with CH-SS. The rate of colonization of the 5-FU CVC was 2.9% (n=12), compared to 5.3% (n=21) in the CH-SS coated catheters (relative reduction in colonization with 5-FU coating of 46%, p=0.055).
Full text >>
Posted by
www.med-centric.com
at
8:08 PM
0
comments
![]()
![]()
Nuvelo Announces Phase 2 SONOMA-3 Trial Did Not Meet Target Product Profile and Discontinues Alfimeprase Development
SAN CARLOS, Calif., March 17 (HSMN NewsFeed) -- Nuvelo, Inc. (Nasdaq: NUVO ) today announced that data from the Phase 2 program in catheter occlusion (CO), known as SONOMA-3 (Speedy Opening of Non-functional and Occluded catheters with Mini-dose Alfimeprase), did not show sufficient improvement in catheter opening at the higher dose and concentration evaluated in the study to meet the desired target product profile. As a result, Nuvelo has ended further clinical development of alfimeprase including its programs in CO and acute ischemic stroke.
Full text >>
Posted by
www.med-centric.com
at
8:05 PM
0
comments
![]()
![]()
Judge Rules in Favor of Cook in Patent Infringement Suit
BLOOMINGTON, Ind.--The United States District Court for the Northern District of California granted summary judgment in favor of Cook Incorporated rejecting claims filed by Edwards Lifesciences LLC alleging Cook had infringed on Edwards’ patents for endovascular devices to treat aneurysms, Cook officials announced today.
press release >> http://www.insideindianabusiness.com/newsitem.asp?ID=28457
Posted by
www.med-centric.com
at
7:59 PM
0
comments
![]()
![]()
Treatment Gives Lung Cancer Patients With Inoperable Tumors Two Years Or More, Study Shows
Posted by
www.med-centric.com
at
7:55 PM
0
comments
![]()
![]()
Wednesday, March 19, 2008
Angiotech Showcases Its Hemostream(TM) Dialysis Catheter At The Society Of Interventional Radiology (SIR) Annual Meeting
Angiotech Pharmaceuticals, Inc. (NASDAQ: ANPI, TSX: ANP) announced that it will introduce and exhibit its innovative HemoStream(TM) chronic dialysis catheter at the 2008 Society of Interventional Radiology (SIR) Annual Scientific Meeting to be held in Washington, DC from March 15-18, 2008."It's exciting to participate at SIR and showcase the addition of another 'best-in-class' device offered by Angiotech. We also anticipate that the HemoStream(TM) could be a great candidate for our combination drug-device technologies, such as Angiotech's innovative 5-FU anti-infective platform," said George Leondis, General Manager, Angiotech Interventional.
Some of the potential benefits of the HemoStream(TM) catheter include:- Patented Triple Arterial Lumen Design: Ensures functional flow rates in the event that two lumens become completely occluded.- Transition: Provides atraumatic over-the-wire insertion without the need for a peel-away sheath.- 360 degree Arterial Tip Configuration: Eliminates catheter "side- walling" against vessel.
In April 2007, Angiotech entered into an agreement with Rex Medical, LP, which granted Angiotech an exclusive license to market and distribute the HemoStream(TM) catheter worldwide. The U.S. Food and Drug Administration (FDA) has given clearance to begin marketing the HemoStream(TM) chronic dialysis catheter in the United States.
full article >> http://www.medicalnewstoday.com/articles/100588.php
Posted by
www.med-centric.com
at
11:07 AM
0
comments
![]()
![]()
Angiotech and Rex Medical announce exclusive licensing and distribution agreement for the "Option(TM)" inferior vena cava filter
VANCOUVER, March 13 /PRNewswire-FirstCall/ - Angiotech Pharmaceuticals, Inc. announced today that it has entered into a definitive licensing agreement with privately held Rex Medical, LP ("Rex Medical") for exclusive worldwide rights to market and distribute the Option(TM) inferior vena cava (IVC) filter, a medical device that is implanted into the body's inferior vena cava to prevent pulmonary embolism (PE).
According to industry research, the U.S. market for IVC filters in 2007 was approximately $200 million with retrievable filters accounting for approximately two-thirds of this market. The Option(TM) IVC filter, developed by Rex Medical, is an IVC filter specifically designed for long-term retrieval post device implantation and is expected to be approved for both permanent and retrievable indications.
full article >> http://money.cnn.com/news/newsfeeds/articles/prnewswire/TO21313032008-1.htm
Posted by
www.med-centric.com
at
11:05 AM
0
comments
![]()
![]()
Cordis Corporation Receives U.S. Food and Drug Administration Clearance for S.M.A.R.T. Nitinol Stent Transhepatic Biliary System for 120 mm and 150 mm
WASHINGTON - (Business Wire) Cordis Corporation announced today at the 33rd Annual Scientific Meeting of the Society of Interventional Radiology meeting it has received 510(k) marketing clearance from the U.S. Food and Drug Administration for the S.M.A.R.T.® Nitinol Stent Transhepatic Biliary System for lengths of 120 mm and 150 mm. These stents are indicated for use in the palliative treatment of malignant strictures in the biliary tree that can restrict the flow of digestive fluids and compromise digestion.
Available for the first time in the US in the new lengths, the stents have demonstrated accurate stent placement, which may decrease the need for additional stents to cover the full narrowing of the bile duct. The safety and effectiveness of this device for use in the vascular system have not been established.
Posted by
www.med-centric.com
at
11:00 AM
0
comments
![]()
![]()
SIR: RF Ablation Extends Survival in Lung Cancer
WASHINGTON, March 17 -- Radiofrequency ablation of small malignant lung lesions appears to offer survival of at least two years for nearly three-quarters of patients not suitable for surgery, a French researcher said here. In 244 patients, 70% were still alive two years after the procedure and 38.8% had no viable malignant lung tissue, according to Thierry de Baere, M.D., of Institut Gustave-Roussy, near Paris.
full article >> http://www.medpagetoday.com/MeetingCoverage/SIRMeeting/tb/8774
Posted by
www.med-centric.com
at
10:58 AM
0
comments
![]()
![]()
Catheter-Directed Thrombolysis Proves Safe in Cancer Patients With Deep Vein Thrombosis: Presented at SIR
WASHINGTON, DC -- March 17, 2008 -- Percutaneous catheter-directed thrombolysis (CDT) is safe for acute symptomatic deep venous thrombosis (DVT) both in cancer patients and in noncancer patients, researchers reported here at the Society of Interventional Radiology (SIR) 33rd Annual Scientific Meeting.Approximately 15% of cancer patients develop DVT, according to lead author Stephen R. Preece, BA, Interventional Radiology Applicant, Johns Hopkins School of Medicine, Baltimore, Maryland.Catheter-directed thrombolysis has been shown to result in rapid reduction of thrombus burden and symptoms, and to reduce the risk of recurrence. However, CDT is associated with greater risk of complications, such as major bleeding, than conventional anticoagulation approaches. Therefore, patients with malignancies are not considered to be good candidates for DVT trials, explained Dr. Preece.
full article >> http://www.docguide.com/news/content.nsf/news/852571020057CCF68525740F006272E7
Posted by
www.med-centric.com
at
9:03 AM
0
comments
![]()
![]()
First Patient Enrolled In SONIC I Registry Of OmniSonics Medical Technologies' OmniWave(TM) Endovascular System
OmniSonics Medical Technologies, Inc., a developer of advanced medical devices for use in the treatment of vascular disease, announced that it has enrolled its first patient in the SONIC I Clinical Registry. SONIC I is a prospective, multi-centered U.S. registry study of the company's OmniWave(TM) Endovascular System (OES) in patients undergoing percutaneous mechanical thrombectomy of acute deep vein thrombosis (DVT). All patients enrolled in the SONIC I are scheduled for follow-up at 30 days and 6 months following the OmniWave procedure. The first patient was enrolled by Daniel Clair, M.D., Chairman of Vascular Surgery at The Cleveland Clinic.
full article >> http://www.medicalnewstoday.com/articles/100869.php
Posted by
www.med-centric.com
at
8:59 AM
0
comments
![]()
![]()
Long-Term Data for 884 Patients Show Vertebroplasty for Osteoporotic Spinal Fractures Provides Dramatic Pain Relief, Greatly Decreases Disability
One’s ability to manage everyday life—such as washing, dressing or standing—was measured by the commonly used Oswestry Disability Questionnaire (ODQ), which was completed by patients before—and again one month after—vertebroplasty. The ODQ scores changed from an average of 69.3 percent +/- 13.5 to 18.8 percent +/- 6.9, showing a highly significant improvement in mobility.
Posted by
www.med-centric.com
at
8:55 AM
0
comments
![]()
![]()
UFE Highly Effective in Cases Where Focused Ultrasound to Treat Uterine Fibroids Failed
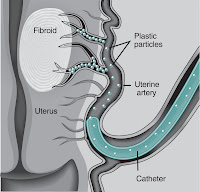 Newswise — Uterine fibroid embolization (UFE), a minimally invasive interventional radiology treatment for uterine fibroids, provides significant relief of symptoms for women whose focused ultrasound (FUS) treatment failed, according to a study released today during the Society of Interventional Radiology’s 33rd Annual Scientific Meeting in Washington, D.C. Primary care physicians and gynecologists can feel confident in informing patients that a failed FUS treatment does not require subsequent gynecological surgery. Those women can be successfully treated with UFE. Patients with a single large fibroid are candidates for FUS; patients with many fibroids, which is more often the case, would be better treated with UFE. There is limited long-term follow-up data for FUS and limited information on fibroid recurrence rates. In this retrospective study from Boston’s Brigham and Women’s Hospital, seven post-FUS patients who experienced therapeutic failure and were subsequently re-treated with UFE were reviewed. In all patients, their symptoms—such as heavy menstrual bleeding and the sensation of fullness or pressure in the lower abdomen—improved
Newswise — Uterine fibroid embolization (UFE), a minimally invasive interventional radiology treatment for uterine fibroids, provides significant relief of symptoms for women whose focused ultrasound (FUS) treatment failed, according to a study released today during the Society of Interventional Radiology’s 33rd Annual Scientific Meeting in Washington, D.C. Primary care physicians and gynecologists can feel confident in informing patients that a failed FUS treatment does not require subsequent gynecological surgery. Those women can be successfully treated with UFE. Patients with a single large fibroid are candidates for FUS; patients with many fibroids, which is more often the case, would be better treated with UFE. There is limited long-term follow-up data for FUS and limited information on fibroid recurrence rates. In this retrospective study from Boston’s Brigham and Women’s Hospital, seven post-FUS patients who experienced therapeutic failure and were subsequently re-treated with UFE were reviewed. In all patients, their symptoms—such as heavy menstrual bleeding and the sensation of fullness or pressure in the lower abdomen—improved
Posted by
www.med-centric.com
at
8:39 AM
0
comments
![]()
![]()
SIR: Percutaneous Cryoablation Effective Against Kidney Cancer
WASHINGTON, March 18 -- Percutaneous cryoablation stops kidney cancer cold, researchers said here. A year after treatment, between 94% and 97% of tumors treated by the minimally invasive technique had not recurred, according to two studies presented at the annual meeting of the Society of Interventional Radiology. The method, which involves freezing the heart of the tumor to about minus 150 degrees Celsius, should now be seen as a "curative minimally invasive treatment for kidney tumors," said Hussein Aoun, M.D., of Wayne State University in Detroit.
full article >> http://www.medpagetoday.com/MeetingCoverage/SIRMeeting/tb/8782
Posted by
www.med-centric.com
at
8:29 AM
0
comments
![]()
![]()
BTG's varicose vein treatment found safe in trial
LONDON, March 17 (Reuters) - British biotech firm BTG Plc's (BGC.L: Quote, Profile, Research) most important pipeline product, a varicose vein treatment called Varisolve, is safe for use in patients with a common heart defect, according to preliminary trial results.
In more than 90 percent of the 28 patients studied so far, tiny bubbles were detected in the blood during the treatment procedure but no neurological, visual or cardiac changes were observed, researchers said on Monday.
The U.S. Phase II safety study will continue until 50 patients have been treated and monitored.
The U.S. Food and Drug Administration requested the trial to see if there was any risk that microbubbles used in Varisolve -- an injectable foam made with carbon dioxide -- could pass into arteries and create problems in the brain.
full article >> http://www.reuters.com/article/rbssHealthcareNews/idUSL1489768220080317
Posted by
www.med-centric.com
at
8:24 AM
0
comments
![]()
![]()
Teleflex Medical Exhibits Arrow Pressure Injectable Acute Central Venous Catheters
RESEARCH TRIANGLE PARK, N.C. - (Business Wire) Teleflex Medical, a leading global supplier of disposable products for critical care and surgical applications, will exhibit its new pressure injectable central venous catheters at the Society of Interventional Radiology Meeting in Washington, D.C. from March 15 - 20. The FDA provided clearance to commercialize Arrow multi-lumen central venous catheters with the additional indication of pressure injection. Arrow is the only manufacturer of acute central venous catheters indicated for pressure injection up to 10ml/second.
Teleflex Medical estimates that nearly 20 percent of patients in acute care settings who are CVC recipients will need a CT scan. The additional indication gives clinicians who perform CT scans more options for scanning patients. CT technicians will now have the option of using an indwelling pressure injectable Arrow CVC without having to insert another catheter just for the scan. Arrow’s additional indication will reduce the stress on patients who up to now have had to endure another catheter insertion.
full article >> http://www.earthtimes.org/articles/show/teleflex-medical-exhibits-arrow-pressure-injectable-acute-central-venous-catheters,319800.shtml
Posted by
www.med-centric.com
at
8:17 AM
0
comments
![]()
![]()
Friday, March 14, 2008
Diomed files for Chapter 11, seeks sale to Biolitec
Diomed Holdings Inc. said Friday it, together with its wholly owned subsidiary, Diomed Inc., has filed a voluntary petition under Chapter 11 for bankruptcy protection.
The petition contemplates that Diomed will sell certain of its operating assets to Biolitec AG, a German-based manufacturer of medical lasers, optical fibers and other products. The move would enable Biolitec to continue to operate Diomed's business in the United States. Diomed develops and markets minimally invasive medical technologies, including a laser treatment for varicose veins.
Andover, Mass.-based Diomed (AMEX: DIO) said it has entered into a non-binding letter of intent with Biolitec for the sale of specified assets for a purchase price of between $6 and $7 million. Biolitec employs approximately 60 people its U.S. operations in East Longmeadow, Mass.
full article >> http://boston.bizjournals.com/boston/stories/2008/03/10/daily46.html?ana=yfcpc
Posted by
www.med-centric.com
at
5:19 PM
0
comments
![]()
![]()
Wednesday, March 12, 2008
Diomed Holdings Gets Delisting Determination Notice From AMEX
Diomed Holdings, Inc. (DIO) said Tuesday that it has received notice from the American Stock Exchange that the AMEX has determined to seek to delist the company's common stock on the basis that the company has not demonstrated a reasonable probability that it will regain compliance with the laid down standards for continued listing on the exchange. The standards require that a company maintain at least $4 million in stockholders' equity if the company has sustained losses from continuing operations in three of its four most recent fiscal years and at least $6 million in stockholders' equity if the company has sustained losses from continuing operations in its five most recent fiscal years.Diomed had previously submitted a compliance plan to the AMEX seeking to show its ability to regain compliance with these listing standards by the AMEX set February 3, 2009 deadline. In its notice, the AMEX also said it has determined that the low trading price of the company' common stock raises concern that the common stock may not be suitable for auction market trading, which would necessitate a reverse stock split within a reasonable period of time under certain section the AMEX Company Guide.
Posted by
www.med-centric.com
at
6:37 PM
0
comments
![]()
![]()
Medtronic Introduces Improvement to Minimally Invasive Treatment of Aortic Aneurysms in Europe
Hydrophilic Coating on Talent(R) Abdominal Stent Graft Aids Navigation of Medical Device through Tight and Tortuous Arteries
MINNEAPOLIS--(HSMN NewsFeed)--Continuing its record of innovation in endovascular therapies for aortic aneurysms, Medtronic, Inc. (NYSE: MDT ), today announced the European market launch of the Talent® Abdominal Stent Graft on the new Xcelerant® Hydro Delivery System, which features a hydrophilic coating designed to aid navigation of the device through tight and tortuous arteries by reducing friction with the artery wall.
full article >> http://salesandmarketingnetwork.com/news_release.php?ID=2023651
Posted by
www.med-centric.com
at
6:31 PM
0
comments
![]()
![]()
Bard Signs Agreement to Acquire Specialized Health Products International, Inc.
MURRAY HILL, N.J.--(HSMN NewsFeed)--C. R. Bard, Inc. (NYSE: BCR ) today announced that it has signed an agreement to acquire all the outstanding shares of Specialized Health Products International, Inc. (OTCBB: SHPI ) for a purchase price of $1.00 per share in cash, totaling approximately $68 million. Bard’s Access Systems subsidiary, located in Salt Lake City, Utah, will assume marketing responsibility for the related products. The company expects to complete the transaction following approval by the shareholders of Specialized Health Products and customary closing conditions, including Hart-Scott-Rodino clearance.
Specialized Health Products manufactures and markets vascular access products, including winged infusion sets, which are used to deliver therapeutic agents through vascular access ports. Many of its devices, including the SafeStep® Huber Needle Set, are designed to reduce the risk of accidental needlesticks for both patients and clinicians. Specialized Health Products is currently an original equipment supplier of winged infusion sets to Bard.
Full text >>
Posted by
www.med-centric.com
at
6:26 PM
0
comments
![]()
![]()
Medegen Introduces MaxPlus(R) Clear, First and Only Clear Positive Displacement Connector for Use in Infusion Therapy
Shown to Enhance Patient Care Leading to Lower Bloodstream Infection and Occlusion Rates
ONTARIO, Calif.--(HSMN NewsFeed)--Maximus, a business unit of leading infusion therapy firm Medegen Inc., today introduced its MaxPlus® Clear positive displacement connector for use in patient care. MaxPlus Clear provides complete visualization of the fluid path providing a visual reminder to completely perform clinical practices such as priming, disinfection, and flushing. This clarity enhances clinical practice which can ultimately reduce the occurrence of bloodstream infections and occlusions in patients receiving infusion therapy.
Maximus is the only medical device manufacturer to offer a clear positive displacement connector.
Full text >>
Posted by
www.med-centric.com
at
6:24 PM
0
comments
![]()
![]()
Genzyme Launches Renvela(R) in the U.S. for Dialysis Patients
Pursues Additional Approvals Globally for Dialysis and Earlier Stage Chronic Kidney Disease Patients
CAMBRIDGE, Mass., March 6 (HSMN NewsFeed) -- Genzyme Corp. (Nasdaq: GENZ ) today announced the U.S. launch of the phosphate binder Renvela® (sevelamer carbonate) for dialysis patients, as well as significant progress in its international efforts to secure additional approvals for the product.
Genzyme has submitted a marketing authorization application to the European Medicines Agency seeking approval of Renvela for the control of serum phosphorus in chronic kidney disease patients regardless of whether they are on dialysis. This application, which includes both tablet and powder formulations, must be validated before it will be accepted for review.
Full text >>
Posted by
www.med-centric.com
at
6:16 PM
0
comments
![]()
![]()
Thursday, March 6, 2008
Ovalum launches micro-catheter
Ovalum Ltd. has launched its TraVerse micro-catheter in the US. It is the firm's first product to be sold. TraVerse is part of the company's CiTOP CiTop Guidewire for peripheral vasculature applications, for which the US Food and Drug Administration (FDA) granted marketing approval in October 2007. CiTOP is used to open blocked arteries in the limbs.
full article >> http://www.globes.co.il/serveen/globes/docview.asp?did=1000317932&fid=942
Posted by
www.med-centric.com
at
10:49 PM
0
comments
![]()
![]()
Contaminant Found in Recalled Heparin
19 people are confirmed dead from allergic reactions associated to heparin use and 785 adverse reports have been linked to heparin since January of last year.
Baxter Healthcare identified the contaminant through sophisticated nuclear magnetic resonance spectroscopy and finds the fake heparin is similar to the blood thinner in its molecular makeup, but not identical.
The company doesn’t know if the contamination is accidental or intentional but it has been found in some five to 20 percent of the active pharmaceuticals in some heparin.
It’s also not known if the contaminant is what has led to so many injuries but the heparin lots associated with illnesses were all found to have the contaminant. What is also still undetermined is whether the Chinese plant is the source of the contamination.
full article >> http://www.injuryboard.com/national-news/contaminant-found-in-recalled-heparin.aspx?googleid=29934
Posted by
www.med-centric.com
at
10:41 PM
0
comments
![]()
![]()
Major Medical Journal Reports Higher Success, Fewer Complications and Lower Cost Treating DVT With Trellis(R) Isolated Thrombolysis
Majority of DVT Cases Treated in Single-Setting With Trellis-8 Catheter From Bacchus Vascular, Reducing Patient Lytic Exposure, Bleeding Complications and Treatment Cost
SANTA CLARA, Calif., March 6 (HSMN NewsFeed) -- Bacchus Vascular Inc., a leading provider of innovative medical devices used by interventional radiologists, vascular surgeons and interventional cardiologists for the minimally invasive treatment of deep vein thrombosis (DVT) and other peripheral vascular disease, announced today that the Journal of Vascular Interventional Radiology (JVIR) has published a study comparing the clinical use of the Trellis-8 infusion catheter from Bacchus Vascular with conventional catheter-directed thrombolysis (CDT) in the treatment of DVT in its March 2008 issue.
Results of the study showed that Grade II and III lysis was achieved in 93% of patients treated with the Trellis catheter and 79% of patients treated with CDT even though thrombolytic doses and infusion durations were less with the Trellis catheter than with conventional CDT. Major hemorrhage was reported in none of the Trellis catheter patients and in 8.5% of patients treated with CDT. The per-patient cost of therapy was $3,697 for the Trellis catheter and $5,473 for CDT. This cost reduction is due to approximately 80% of the Trellis catheter patients being treated in the single-setting of the interventional suite with less time required for follow-up monitoring in a costly critical care unit compared with CDT patients.
Full text >>
Posted by
www.med-centric.com
at
10:29 PM
0
comments
![]()
![]()
Cardinal Health Agrees to Acquire ChloraPrep(R) Manufacturer for $490 Million
Expands Offerings to Help Hospitals Prevent Infections
DUBLIN, Ohio, March 4 (HSMN NewsFeed) -- Cardinal Health, a global provider of products and services that improve the safety and productivity of health care, today announced a definitive agreement to acquire the assets of privately held Enturia Inc. for $490 million. The cash transaction includes Enturia's leading line of infection prevention products sold under the ChloraPrep® brand name and is expected to close within 60 days, subject to customary regulatory approvals and other conditions.
Full text >>
Posted by
www.med-centric.com
at
10:16 PM
0
comments
![]()
![]()
Sunday, March 2, 2008
Hatch Medical to Broker Chronic Total Occlusion Device
DULUTH, Ga.--(HSMN NewsFeed)--Hatch Medical, L.L.C., a medical device incubator and technology brokerage firm, announced today that it has recently entered into an agreement with Medical Miracles (Leeds, UK) to broker its patented and CE Marked chronic total occlusion (CTO) device, POLAR™.
POLAR™ (Path Of Least Arterial Resistance) is a mechanical device that generates reciprocal and lateral movements at the distal end of standard guidewires with frequencies of 16 to 100 Hz. when passed through an angioplasty balloon catheter, allowing clinicians to confidently cross occluded vessels.
Full text >>
Posted by
www.med-centric.com
at
11:13 PM
0
comments
![]()
![]()
First Patient Enrolled in Study Evaluating Performance of Spectranetics Laser Ablation Followed by GORE VIABAHN(R) Endoprosthesis...
Physician-sponsored study to examine combination therapy for lower limb in-stent restenosis in patients treated for Peripheral Vascular Disease
FLAGSTAFF, Ariz. & COLORADO SPRINGS, Colo.--(HSMN NewsFeed)--W. L. Gore & Associates, Inc. (Gore) and Spectranetics (NASDAQ: SPNC ) today announced the enrollment of the first two patients in the VIVA II: SALVAGE Trial. The patients were treated by Dr. Eric Dippel at Midwest Cardiovascular Research in Davenport, Iowa. The physician-sponsored SALVAGE Trial, is designed to evaluate the safety and performance of the GORE VIABAHN® Endoprosthesis with Heparin Bioactive Surface and the Spectranetics TURBO-Booster® and TURBO elite® laser catheter with the CVX-300® Excimer Laser System for the treatment of peripheral vascular disease (PVD) in the superficial femoral artery (SFA). Specifically, the study will evaluate the effectiveness of this combination therapy as a treatment for patients with chronic lower-limb ischemia associated with femoro-popliteal in-stent restenosis.
Full text >>
Posted by
www.med-centric.com
at
11:03 PM
0
comments
![]()
![]()
Wednesday, February 27, 2008
Vnus Medical Reveals Positive Clinical Data For VNUS ClosureFAST Catheter At American Venous Forum In South Carolina
(RTTNews) - Vnus Medical Technologies, Inc. on Saturday said it presented positive clinical performance data for the Vnus ClosureFAST catheter at the annual meeting of the American Venous Forum in Charleston, South Carolina. The data affirmed the efficacy and patient comfort levels of endovenous radiofrequency vein ablation using Vnus ClosureFAST.
full article >> http://www.tradingmarkets.com/.site/news/BREAKING%20NEWS/1128710/
Posted by
www.med-centric.com
at
8:39 AM
0
comments
![]()
![]()
Veinlite by TransLite Improves Vein Access in Children
SUGAR LAND, Texas, Feb. 26, 2008 (PRIME NEWSWIRE) -- A new publication by researchers at the Boston Children's Hospital shows that the use of Veinlite(r) transillumination improves vein access in children from 74% to 85% in the pediatric emergency department.
Vein access is a common and necessary procedure that is used to draw blood and infuse fluids in patients. Children have small veins that are hard to access, often resulting in multiple tries which increases trauma to the patient and parent. Failures to access veins often require invasive procedures that increase the risk of morbidity to the patient and increase the cost of treatment.
full article >> http://www.primenewswire.com/newsroom/news.html?d=137135
Posted by
www.med-centric.com
at
8:34 AM
0
comments
![]()
![]()
RadSciences Group announces release of 2008 Radiology Compensation Review
RadSciences Group is releasing the results of the most recent Radiology Compensation Review that focuses on real-time wage changes and staffing trends that have affected radiology groups and hospitals over the past year, including their recruitment and retention efforts. The Compensation Review is a free resource that provides a summary of current data and market trends in all modalities of radiology. The latest figures show a continued market demand for sonographers, vascular sonographers, cardiac sonographers and interventional technologists. The number of General, vascular and cardiac sonographer searches conducted by RadSciences Group increased from 47.9 per cent of overall searches in 2006 to 58.5 per cent in 2007.
full article >> http://www.medicexchange.com/mall/departmentpage.cfm/MedicExchangeUSA/_81675/3864/departments-contentview
Posted by
www.med-centric.com
at
8:32 AM
0
comments
![]()
![]()
Friday, February 22, 2008
Medical Device Ruling Redraws Lines on Lawsuits
The Supreme Court’s decision Wednesday protecting many types of medical device makers from personal injury lawsuits began rippling through the courts and law offices almost immediately.
Hours after the decision in the case, Riegel v. Medtronic, was announced, lawyers involved in a group of Florida state court cases related to Johnson & Johnson’s drug-coated Cypher heart stent received an e-mail message from Judge Mary Barzee Flores asking for briefs on whether the lawsuits should be allowed to continue.
And lawyers for patients with injuries they attribute to other devices like heart valves, artificial hips and defibrillators said they were girding for a flood of court filings from device makers like Medtronic asking judges to dismiss such lawsuits.
full article >> http://www.nytimes.com/2008/02/22/business/22device.html?ref=business
Posted by
www.med-centric.com
at
12:17 AM
0
comments
![]()
![]()
Wednesday, February 20, 2008
AngioDynamics Announces Departure of COO Robert Mitchell
Mitchell Accepts CEO Position of Start-up Medical Device Company
QUEENSBURY, N.Y. February 19, 2008—AngioDynamics (NASDAQ: ANGO), a leading provider of innovative medical devices used by interventional radiologists, nephrologists, and surgeons for the minimally invasive treatment of cancer and peripheral vascular disease, announced today that Robert D. Mitchell, the Company’s Chief Operating Officer, has resigned his position effective February 29, 2008 to become the CEO of Nellix Endovascular, a start-up medical device company.
“I thank Bob Mitchell for his efforts and contributions over the past 14 months and wish him well as he pursues the next chapter of his career,” said Eamonn Hobbs, President & CEO. “Over the past year, we have strengthened our senior management team. While I will be assuming many of Bob’s responsibilities, others will add some responsibilities. We expect a smooth transition and a continuation of our recent positive momentum.”
full article >> http://www.angiodynamics.com/pages/news/news_articles_detail.asp?id=244
Posted by
www.med-centric.com
at
8:42 AM
0
comments
![]()
![]()
Thursday, February 14, 2008
Paclitaxel-coated balloon reduces restenosis after leg angioplasty
Boston, MA - A paclitaxel-coated balloon may end up filling a role never successfully filled by drug-eluting stents (DES): angioplasty of the leg. According to Dr Gunnar Tepe (Eberhard-Karls-Universitat, Tubingen, Germany) and colleagues, use of a paclitaxel-eluting balloon was significantly more effective in terms of reducing need for revascularization than either a bare, uncoated balloon or a bare balloon coupled with paclitaxel-infused contrast medium [1].
full article >> http://www.theheart.org/article/843165.do
Posted by
www.med-centric.com
at
10:22 AM
0
comments
![]()
![]()
Merit Finalizes Two Deals
SOUTH JORDAN, Utah, Feb. 13, 2008 (PRIME NEWSWIRE) -- Merit Medical Systems, Inc. (Nasdaq:MMSI), a manufacturer and marketer of proprietary disposable devices used primarily in cardiology and radiology procedures, announced today that the Company has finalized two transactions.
On November 5, 2007, Merit announced that it had entered a non-binding term sheet to acquire cardiac and peripheral catheter platform assets from Micrus Endovascular. On January 31, 2008, Merit finalized a definitive asset purchase and supply agreement effecting the transaction.
Additionally, the Company said that is has entered into an agreement with Timothy Clark, M.D., to acquire intellectual property rights relating to U.S. Patent #7,087,060, Methods for Obtaining Hemostasis of Percutaneous Wounds.
full article >> http://money.cnn.com/news/newsfeeds/articles/primenewswire/136283.htm
Posted by
www.med-centric.com
at
10:02 AM
0
comments
![]()
![]()
Terumo Interventional Systems Expands Its Renowned Glidewire® Hydrophilic Coated Guidewire Product Line
February 12, 2008 -- Somerset, New Jersey -- With its unique double angle to facilitate the selection of vessels that lie at, or originate at, difficult to access angles and take-offs, the Bolia curve is among the new additions to the Terumo Glidewire Hydrophilic Coated Guidewire product line.
In a continuous effort to provide physicians with new tools to help ensure procedural success, Terumo Interventional Systems has expanded its Glidewire product line to include 11 new guidewire configurations including a brand new tip shape design, the Bolia curve.
The Bolia curve, named for Dr. Aman Bolia who helped Terumo develop this unique tip shape, is designed to facilitate selection of vessels that are difficult to access.
Already having an extensive selection of hydrophilic coated nitinol wires, including a wide choice of 0.018” and shapeable tip Glidewires, Terumo has further expanded its offering as a response to customers’ requests. The additions include the unique 1 cm taper, available in different lengths and stiffness, as well as an 80 cm 0.035” and 0.038” Glidewire.
full article>> http://www.ptca.org/news/2008/0212_TERUMO.html
Posted by
www.med-centric.com
at
10:00 AM
0
comments
![]()
![]()
Tuesday, February 12, 2008
Bayer to Buy Possis
German pharmaceutical and chemical company Bayer AG agreed to buy Possis Medical Inc. in a $361 million deal that will help Bayer expand its footprint in the field of cardiovascular health technology.
The all-cash deal, set to close later this quarter, is for $19.50 a share, a 36% premium to Friday's closing price for Possis of $14.35. The stock hasn't traded at $19.50 since 2004.
The proposed combination of Bayer's Medrad subsidiary and Possis will join a provider of systems used to diagnose cardiovascular and other diseases with a maker of devices used to treat narrowed or blocked arteries.
full article >> http://online.wsj.com/article/SB120273475957358689.html?mod=googlenews_wsj
Posted by
www.med-centric.com
at
8:53 AM
0
comments
![]()
![]()
Cook Medical Enrolls First U.S. Patient in STABLE Aortic Dissection Trial
Leading Vascular Surgeons Treat First U.S. Patient in Groundbreaking Trial at Thomas Jefferson University Hospital
BLOOMINGTON, Ind.--(BUSINESS WIRE)--Cook Medical today announced enrollment of the first U.S. patient in the STABLE global clinical trial designed to evaluate the Cook Zenith® Dissection Endovascular System for the treatment of Type B thoracic aortic dissections. This is the first device designed specifically to treat aortic dissections, the condition that took the life of actor John Ritter. The technologically advanced device is the first-of-its-kind worldwide and was designed to treat the unique morphology of this disease, a major cause of mortality worldwide.
press release >> http://www.businesswire.com/portal/site/google/index.jsp?ndmViewId=news_view&newsId=20080211005332&newsLang=en
Posted by
www.med-centric.com
at
8:46 AM
0
comments
![]()
![]()
Manufacturer Halts Production of Blood Thinner Heparin
MONDAY, Feb. 11 (HealthDay News) -- Baxter HealthCare Corp., which produces half of the blood thinner heparin sold in the United States, said Monday that it was temporarily suspending production of its multi-dose injectable form of the drug following reports of serious allergic reactions and possibly four patient deaths.
The cause of the allergic reactions isn't known. It's also not clear how long production will be stopped. The one thing that is clear is that the stoppage could lead to a shortage of heparin, which is used to prevent blood clots in such patients as those undergoing kidney dialysis and heart surgery, U.S. health officials said.
Since the end of December, there have been about 350 reports of adverse reactions associated with Baxter's heparin product. This compares with less than 100 reports of adverse reactions in all of 2007, Dr. John Jenkins, director of the U.S. Food and Drug Administration Office of New Drugs at the Center for Drug Evaluation and Research, said during a Monday teleconference.
full article >> http://www.forbes.com/forbeslife/health/feeds/hscout/2008/02/11/hscout612578.html
Posted by
www.med-centric.com
at
8:42 AM
0
comments
![]()
![]()
Intermediate comparison of partial nephrectomy and radiofrequency ablation for clinical T1a renal tumors
Urol Oncol. 2008 Jan-Feb;26(1):102.
Stern JM, Svatek R, Park S, Hermann M, Lotan Y, Sagalowsky AI, Cadeddu JA, Department of Urology, University of Texas, Southwestern Medical Center, Dallas, TX.
Boorjian SA, Blute ML.
OBJECTIVE: To compare the intermediate term outcomes of patients with clinical T1a renal tumors who were treated with nephron-sparing surgery by partial nephrectomy (PN), the preferred approach for small (cT1a) renal tumors, or radiofrequency ablation (RFA), recently offered to selected patients as an alternative, less morbid technique. PATIENTS AND METHODS: We identified patients with stage T1a renal masses who had >/=2 years of follow-up; those with bilateral synchronous or metachronous tumors, metastatic disease at presentation, or a family history of renal cell carcinoma were excluded. From July 1996 to January 2004, 110 PNs were identified in our database; 37 patients who fulfilled the inclusion criteria had either open (30) or laparoscopic PN (7), and 40 had either percutaneous (26) or laparoscopic (14) RFA. RESULTS: The mean (range) follow-up for the RFA and PN groups was 30 (18-42) and 47 (24-93) months, respectively; the respective mean tumor size was 2.41 and 2.43 cm. There was 1 incomplete ablation and 2 local recurrences in the RFA group, and 2 recurrences in the PN group (1 local and 1 in the contralateral kidney). There were no disease-specific deaths. The overall actuarial disease-free probability for the PN and RFA groups, respectively, was 95.8% and 93.4% (P = 0.67). CONCLUSIONS: This initial 3-year actuarial analysis showed that RFA for cT1a renal tumors has comparable oncological outcomes to PN; however, longer term data are still needed.
Posted by
www.med-centric.com
at
8:37 AM
0
comments
![]()
![]()
Sunday, February 10, 2008
ev3 Inc. Accepts Resignation of Dr. John Simpson
PLYMOUTH, Minn., Feb 08, 2008 /PRNewswire-FirstCall via COMTEX/ -- ev3 Inc. (Nasdaq: EVVV), a global endovascular device company, today announced the resignation of Dr. John Simpson from his position as Chief Scientist and from the company's board of directors effective February 7, 2008.
"Over the last thirty years, few people have shaped the treatment of cardiovascular disease as much as Dr. John Simpson with his numerous inventions and vision for improving the lives of patients worldwide," said Jim Corbett, chairman, president and chief executive officer of ev3 Inc. "He leaves a legacy of innovation and a foundation on which we intend to build in our effort to improve patient treatment."
full article >> http://www.foxbusiness.com/markets/industries/health-care/article/ev3-accepts-resignation-dr-john-simpson_472442_10.html
Posted by
www.med-centric.com
at
6:28 PM
0
comments
![]()
![]()
Early-stage firm applies microwave power to cancer tumors
Madison, Wis. - NeuWave Medical, a Madison-based medical device company that has landed $4.5 million in venture capital to further develop a microwave probe that destroys tumors, will try to capture a global market conservatively estimated at more than $200 million, according to one of its founding researchers. Daniel van der Weide, who developed the new microwave technology to ablate, or destroy cancerous tumors along with his University of Wisconsin-Madison colleague Fred T. Lee Jr, said there are a number of potential applications for the device, a minimally invasive microwave probe that is inserted into a patient like a needle to deliver microwaves that destroy tumors.
full article >> http://wistechnology.com/article.php?id=4529
Posted by
www.med-centric.com
at
6:20 PM
0
comments
![]()
![]()
Friday, February 8, 2008
Endovenous 980-nm laser treatment of saphenous veins in a series of 500 patients
J Vasc Surg. 2007 Dec;46(6):1242-7.
Desmyttère J, Grard C, Wassmer B, Mordon S.
S.E.L. Angéio-Phlébo Interventionnelle, France.
BACKGROUND: In recent years, endovenous laser treatment (ELT) has been proposed to treat incompetent great saphenous veins (GSV). This study reports the long-term outcome of ELT in a series of 500 patients. METHODS: Incompetent GSV segments in 500 patients (436 women, 64 men) with a mean age of 52.6 years (range, 19 to 83 years) were treated with intraluminal ELT using a 980-nm diode laser (Pharaon, Osyris, France). The GSV diameter was measured by Duplex examination in an upright position in different GSV segments (1.5 cm below the saphenofemoral junction, crural segment, condylar segment, and sural segment). These measurements were used to determine the optimal linear endovenous energy density (LEED) for each segment. During treatment, patients were maintained in the Trendelenburg position. Patients were evaluated clinically and by duplex scanning at 1 and 8 days, 1 and 6 months, and at 1, 2, 3, and 4 years to assess treatment efficacy and adverse reactions. RESULTS: A total of 511 GSVs were treated. The mean diameter was 7.5 mm (range, 2.4 to 15.0). The LEED was tuned as a function of the initial GSV diameter measured in the orthostatic position, from 50 J/cm (3 mm) up to 120 J/cm (15 mm). At the 1-week follow-up, 9.3% of the patients reported moderate pain. In the immediate postoperative period, the closure rate was 98.0% and remained constant during the 4-year follow-up to reach 97.1%. After 1 year, a complete disappearance of the GSV or minimal residual fibrous cord was noted. Major complications have not been detected; in particular, no deep venous thrombosis. Ecchymoses were seen in 60%, transitory paresthesia was observed in 7%. There was no dyschromia, superficial burns, thrombophlebitis, or palpable indurations. Complementary phlebectomy was done in 98% of patients. Failures occurred only in large veins (saphenofemoral junction diameter >1.1 cm or for GSV truncular diameter >0.8 cm) CONCLUSION: ELT of the incompetent GSV with a 980-nm diode laser appears to be an extremely safe technique, particularly when the energy applied is calculated as a function of the GSV diameter. It is associated with only minor effects. Currently, ELT has become the method of choice for treating superficial veins and has almost replaced the treatment of traditional ligation and stripping.
Posted by
www.med-centric.com
at
8:09 PM
1 comments
![]()
![]()




